July 29, 2020 video: Importation Executive Order Explainer
On July 24, 2020, the White House issued an executive order to implement three approaches to foreign drug importation. To be clear, this isn’t normal importation of medicine. Food and Drug Administration (FDA)-regulated manufacturers routinely make medicines in other countries to be sold on the U.S. market. The executive order, however, involves dipping into other countries’ drug supplies and putting them in U.S. medicine cabinets.
Before this announcement there were two pathways outlined in draft regulations for importing medicine from other countries drug supplies:
- Pathway #1: State-sponsored importation programs from Canadian suppliers that must be approved by HHS.
- Pathway #2: Manufacturer-sponsored importation of their own products into the United States.
The July 24, 2020 executive order reaffirmed the White House’s commitment to completing the regulations for Pathway #1, and created two new pathways: individual waivers for personal importation and insulin for emergency medical care.
Individual Waivers (Pathway #3)
The first importation option listed in the order references section 804(j)(2) of the Food, Drugs and Cosmetics Act (FDCA), which allows the U.S. Department of Health and Human Services (HHS) to grant individuals waivers—either on a case-by-case basis or by regulation—to import medicine and medical devices for personal use. The next paragraph of the law, 804(j)(3), specifies that any imported medicine must be:
- Imported with a valid prescription from a licensed pharmacy in Canada, from a seller registered with HHS.
- An FDA-approved drug manufactured in an FDA-approved facility sold in the final form that the FDA has permitted.
- Imported under conditions that HHS has determined ensures public safety.
- No more than a 90-day supply.
Although importation proponents have asserted that the FDCA permits personal importation in the past, the actual code shows how narrow that permission is: importation is only legal if the HHS secretary has granted a specific waiver; the patient must buy from a list of HHS-approved sellers who are licensed in Canada, and the drug being imported must be FDA-approved and manufactured in a facility registered with the FDA. Brokers taking orders on a website with a Canadian flag and filling them with drugs from all over the world were not legal on July 23rd. And they’re still not legal today.
Insulin for Emergency Medical Care (Pathway #4)
The second part of the executive order directs the Secretary of HHS to use their powers to bring insulin in from other countries under the authorization granted for “emergency medical care” in Section 801(d)(2) of the FDCA. Access to insulin is unquestionably critical for the more than 8.2 million U.S. residents who have insulin-dependent diabetes. As the government of Canada has noted, however, Canada accounts for only two percent of global drug sales, and the United States accounts for 44 percent, and Canada is not in a position to increase its share of medicines to sell them to the U.S. If Canada were to allow the U.S. import insulin in bulk from our northern neighbors it would cause drug shortages and Canadian deaths.
ran 20-to-1 against the proposal. Learn more.
Finish the Rulemaking for State Importation (Pathway #1)
The final part of the executive order directs HHS to complete the rulemaking posted in December 2019 that would allow wholesalers, pharmacists, and by proxy, states to import Canadian medicine in bulk. PSM examined the proposed rulemaking and concluded that it would increase the risk of members of the U.S. public receiving counterfeit, adulterated, misbranded, or otherwise unsafe medicines. We also reviewed more than 1200 public comments posted in response to it once the comment period had closed and found that members of every part of the pharmaceutical supply chain in the U.S. and Canada thought that state importation was a bad idea that, as the Healthcare Distribution Alliance wrote this week, would have “significant implications for the safety and security of the pharmaceutical supply chain and patient health.”
We Cannot Solve This Problem by Raiding Canada’s Drug Supply
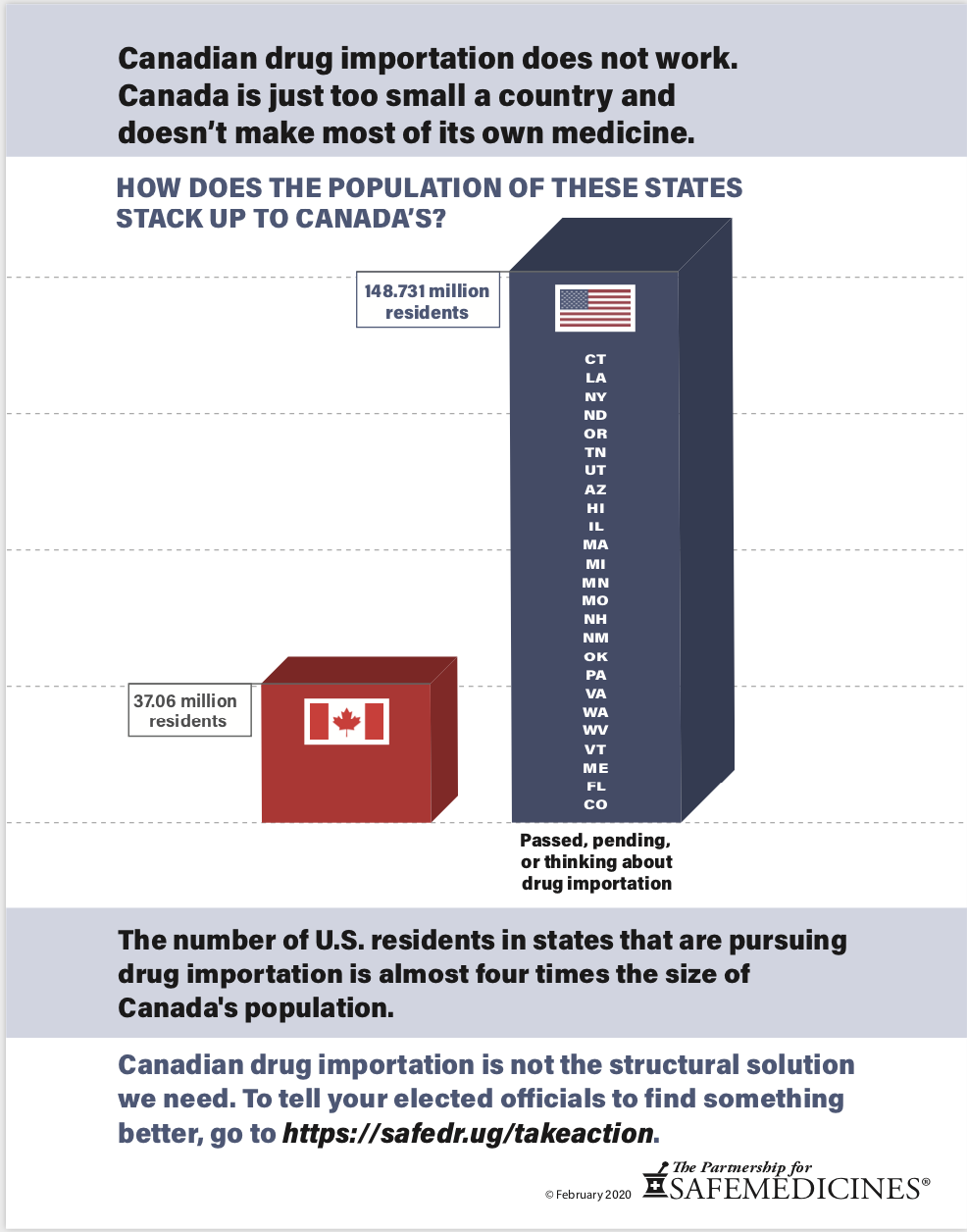
Canada’s population is one-ninth the size of ours and it already faces substantial drug shortages. 65% of their drug supply is manufactured overseas, so they cannot simply increase their production to accommodate the needs of U.S. residents. The Canadian prescription drug supply would last approximately 182 days if twenty percent of U.S. prescriptions came from Canada.
For 18 years, no head of HHS or FDA under any president has endorsed importing Canadian drugs, and many previous FDA commissioners and HHS secretaries have gone on record against it. Law enforcement, patient groups, and healthcare professionals from every province in Canada and all over the U.S. oppose importation.
Illinois, Maine, and Minnesota have tried rogue Canadian importation programs in the past with poor results in terms of safety and cost. There is no reason to believe that they will fare better now. States across the entire country are facing critical budget shortfalls because of the COVID-19 pandemic, but the White House is telling states to move forward with drug importation anyway. Florida has already allocated eleven million dollars to fund importation programs while gutting programs that would have brought drinkable water to Pasco County, raised pay for caregivers to the disabled, and treated inmates with contagious and deadly hepatitis C. In Colorado, importation is being considered alongside important education, housing and healthcare initiatives. All this, despite the fact that our Canadian counterparts have rejected the idea.
Sources for this week's video and blog
- 21 USC 381: Imports and Exports
- 21 USC 384: Importation of Prescription Drugs
- Former FDA Commissioners, Drs. Robert Califf, Margaret B. Hamburg, Mark B. McClellan, and Andrew Von Eschenbach, Letter to Congress, March 16, 2017
- “Comments on the Proposed Rule, ‘Importation of Prescription Drugs’, Docket No. FDA-2019-N-5711” The Government of Canada.
- Drug Shortages Canada
- "Executive Order on Increasing Drug Importation to Lower Prices for American Patients," The White House, July 24, 2020.
- "Importation of Prescription Drugs, Proposed Rulemaking," Docket No. FDA-2019-N-5711, December 18, 2019.
- United States Diabetes Surveillance System, Centers for Disease Control.
- "Presidential Executive Orders Will Not Lower Drug Costs," Citizens Against Government Waste, July 28, 2020.
- "Statement in Response to U.S. Drug Importation Legislation," Diabetes Canada, July 26, 2019.
- John Frank, “Budget Crunch Puts Colorado Democratic Accomplishments at Risk,” The Colorado Sun, April 30, 2020.
- Former FDA Commissioner Dr. Scott Gottlieb, Remarks on Drug Importation, National Press Club, C-SPAN, May 10, 2019.
- “HDA Statement on the President's Drug Affordability Executive Orders,” Healthcare Distribution Alliance, July 24, 2020.
- Carl Lisciandrello and Mark Schreiner, “Gov. DeSantis Vetoes $1 Billion From Florida Budget, Citing 'Difficult Circumstances',” WUSF News, June 29, 2020.
- Elizabeth McNichol and Michael Leachman, “States Continue to Face Large Shortfalls Due to COVID-19 Effects,” Center on Budget and Policy Priorities, July 7, 2020.
- Marv Shepherd, “New Pathways for U.S. Importation Threaten Canadian Prescription Drug Supply,” Canadian Health Policy, September 2019.
- Jim Turner, “Ron DeSantis Gets Florida Budget, Ponders Cuts,” Tampa Bay Times, June 17, 2020.
Resources from The Partnership for Safe Medicines:
- "Drugs From Online Canadian Pharmacies Come From Many Places—But Not Canada," April 2018.
- “Eight Surprising Things We Learned from Reading the 1,200 Comments Filed with the FDA on Canadian Drug Importation,” March 23, 2020.
- "HHS Comments Come In Overwhelmingly Against Canadian Drug Importation Proposal," March 2020.
- “In 2013, Maine Became The 1st State In The Country To Enact A ‘Drug Importation Ordinance,’” November 6, 2015.
- “Illinois’ Experience With ISaveRX, 2004–2009,” November 6, 2015.
- “Minnesota’s Experiment With Drug Importation: RxConnect 2003-2010,” March 2019.
- “PSM Files Comments On HHS's Proposed Canadian Drug Importation Rules; Cites Danger To Patients,” February 11, 2020.
- “What Canadians Are Saying About Drug Importation, January 2019 - December 2019.”
- "Who Opposes Canadian Drug Importation?" 2018.