FDA Alert: Dietary Supplement For Vision Care Recalled Due to Unapproved and Dangerous Ingredients
This is a reprint of an FDA Alert.
TruVision Health Recalls Dietary Supplement Products Because of Possible Health Risk
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
May 2, 2023
Summary
Company Announcement Date: April 27, 2023
FDA Publish Date: April 27, 2023
Product Type: Food & Beverages
Reason for Announcement: Unapproved Hordenine and octodrine/DMHA (1,5-dimethylhexylamine)
Company Name: TruVision Health
Brand Name: Truvy, TruVision, reFORM
Product Description: Various Dietary Supplement Capsules
Company Announcement
TruVision Health LLC is recalling the dietary supplement products listed below because they contain the unapproved dietary ingredients hordenine and/or octodrine/DMHA (1,5-Dimethylhexylamine).
Hordenine is possibly unsafe when taken by mouth and might cause stimulating side effects such as rapid heart rate, high blood pressure, jitteriness, nervousness, nausea, vomiting or insomnia. These adverse events are more likely to occur in sensitive sub-populations of people such as pregnant women and consumers with cardiovascular disease. Currently, hordenine is not an approved dietary ingredient in dietary supplements.
Octodrine or DMHA (1,5-Dimethylhexylamine) appears to be similar to another stimulant called dimethylamylamine (DMAA), which was removed from the market in certain countries due to safety concerns. In animal studies, octodrine has been found to increase heart rate, myocardial contractility, and pain threshold. Since no data exists on its metabolic pathway in humans, the use of octodrine during exercise is potentially dangerous. DMHA is considered to be a substance that does not meet the statutory definition of a dietary ingredient and is an unsafe food additive.
You should stop using the recalled product(s) immediately.
Some consumers of these products have reported experiencing chest pain, chills, diarrhea, dizziness/lightheadedness, fatigue, headache, high blood pressure, high heart rate, jitters, nausea, nervousness, rash, stomach pain or upset, sweating and vomiting.
Products affected are:
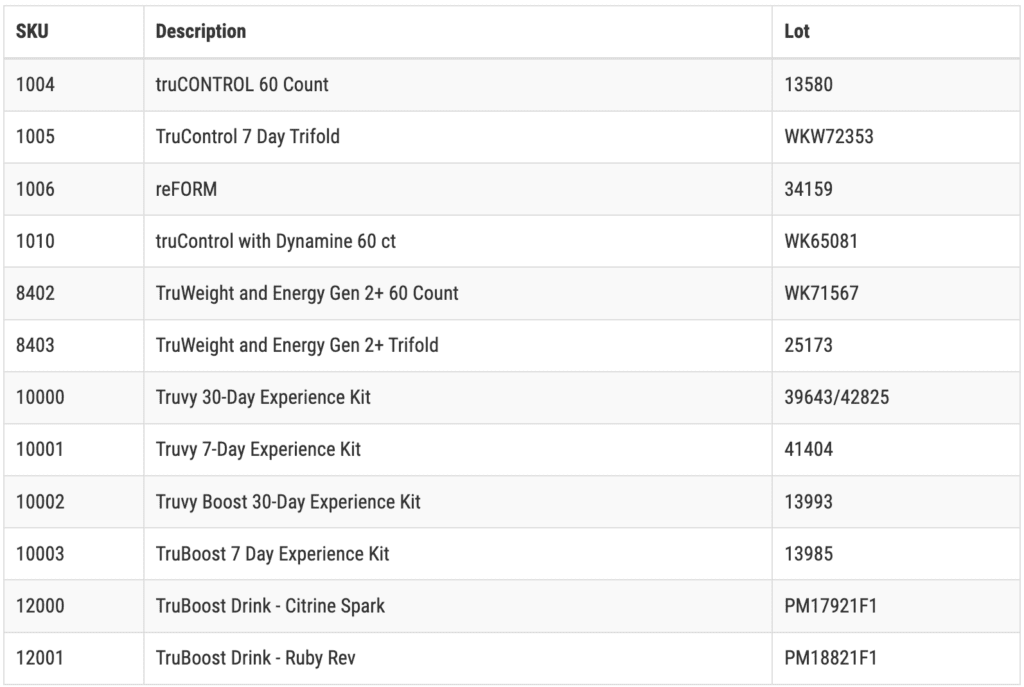
These products were packaged as capsules in blister packs and cardboard cartons or as stick packs in 30 count bags. The product bears the TruVision Health or the Truvy brand name. Lot numbers are located on the end flap of the carton or the back of the bag. The products were sold online at Truvy.com to retail customers and received though US postal service or a parcel carrier like UPS. The products were distributed throughout the US, Canada, Australia, New Zealand, Germany, Ireland and England.
Consumers who have purchased these products should stop using them immediately and they are urged to return them to TruVision Health LLC for a full refund (return shipping is paid by the consumer), an exchange, or they may discard the product. Consumers with questions may contact Truvy Customer Support by calling (855) 213-8788, Monday-Friday from 7:00 am – 6:00 pm MDT.
This recall was initiated after an FDA inspection determined that the products were adulterated.
This recall is being made with the knowledge of the Food and Drug Administration.
Company Contact Information
Consumers:
Truvy Customer Support
(855) 213-8788





