September 8, 2025: FDA launches an import alert listing safe GLP-1 manufacturers
Major Stories
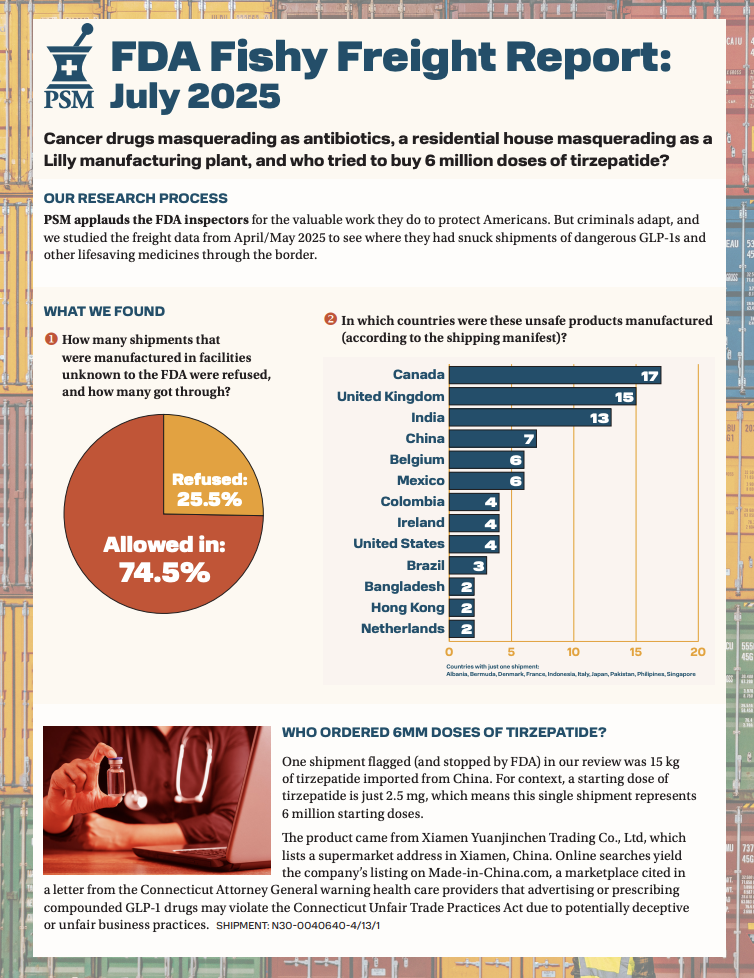
On September 5, the U.S. Food and Drug Administration (FDA) launched a green list import alert that identifies safe sources of active GLP-1 ingredients. As Politico's AgencyIQ pointed out in its FDA Today: Life Sciences newsletter, the FDA is responding to a July 25 letter from legislators and PSM’s July Prescription Drug Freight Fraud Report, which identified dozens of shipments of GLP-1s entering the U.S. from facilities not registered with the FDA. The green list will help protect patients from unreliable, illegally compounded GLP-1s and simplify screening for border security, as GLP-1s from sources that are not on the list will be subject to detention without physical examination.
The FDA’s 2026 legislative proposals include a policy that would simplify the process even further by requiring the destruction of non-compliant, imported products that present a risk to public health. On September 4, PSM signed a letter of support for that proposal, which would prevent rogue sellers from reshipping the very same dangerous medicines border agents had already turned away.
Read PSM's July "Fishy Freight" report
Domestic News
135 organizations press the U.S. legislature for PBM reform. The FDA warned seven companies to stop selling unapproved eye drops.
PSM was one of 135 organizations that signed a letter urging Speaker of the House Mike Johnson, House Democratic Leader Hakeem Jeffries, Senate Majority Leader John Thune, and Senate Democratic Leader Chuck Schumer to protect U.S. pharmacies by passing legislation to address the “anti-competitive, anti-consumer practices” of pharmacy benefit managers. Read the letter, and learn about this issue on our PBM page.
A federal grand jury in Ohio indicted four Chinese pharmaceutical companies and 25 individuals, alleging that they presented themselves as online pharmacies or chemical companies selling legitimate pharmaceuticals, while deliberately supplying U.S. drug dealers with substances such as protonitazene, metonitazene, medetomidine, and xylazine to increase the yield and potency of fentanyl they were distributing. Working in parallel, the U.S. Department of the Treasury’s Office of Foreign Assets Control sanctioned one of the companies, Guangzhou Tengyue Chemical Co., Ltd., and two of its employees.
The Grover Lab at the University of California, Riverside released a preprint of “Disintegration Fingerprinting: A low-cost and easy-to-use tool for identifying substandard and falsified medicines.” The paper, which is not yet peer reviewed, describes a technique to identify solid-dosage drugs based on how they dissolve in liquid. Dr. William Grover, who leads the Grover Lab, spoke to PSM about other innovative ways to detect fake medicines in our July 2025 podcast.
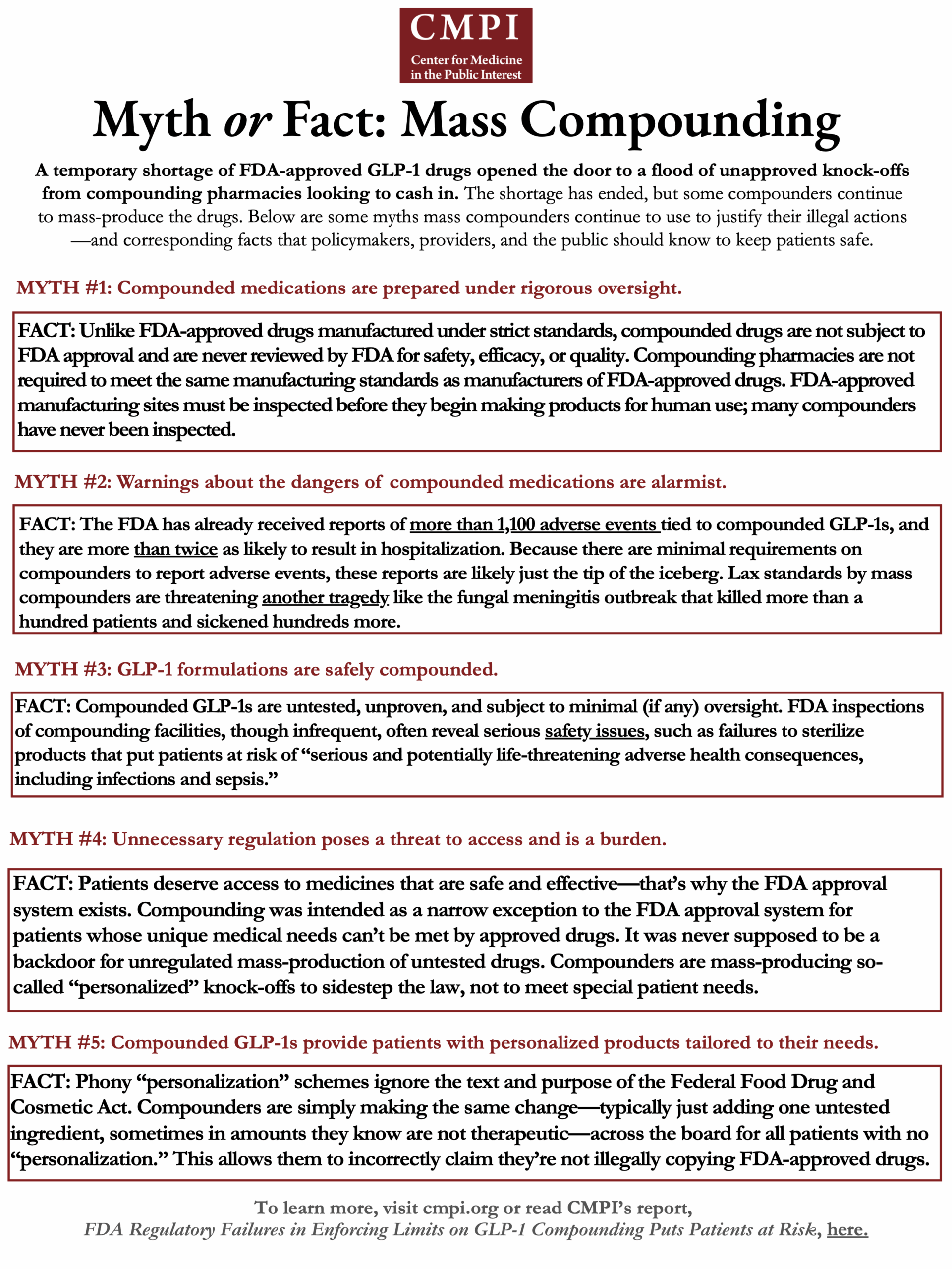
The Center for Medicine in the Public Interest has published a handout to dispel myths about mass compounding. Read it here.
A Lawrence, Massachusetts man was charged with the possession of counterfeit pills after law enforcement discovered multiple counterfeit Adderall and Percocet pills, a pill press, and bowls and bags full of suspected fentanyl and methamphetamine powders during a search of his home and vehicle.
The FDA posted warning letters it sent to My Holistic Honey, Green Vitality, Mel Honey US, Life Enthusiast Co-op, Homeopathic Educational Services, Trew Balance, and Supply Center USA for selling non-FDA approved eye drops. The agency has repeatedly issued warnings about contaminated, unapproved, and adulterated eye drops in recent years, including an October 2023 communication about 26 over-the-counter eye drop products made in unsanitary conditions.
The FDA also warned manufacturing facilities in Wisconsin and New York, and a Colorado compounder of veterinary drugs for violations of Current Good Manufacturing Practice.
Legislation
Keep up with state legislation in the areas of pill presses, prescription drug affordability boards, and drug importation.
Patient safety issues in the GLP-1 space this week
Reddit, September 2, 2025 (Click the image to enlarge it)
Although online searches turn up many businesses selling what they call GLP-1 drops for people who wish to avoid injections, there is no evidence that these products contain GLP-1s or that they would be effective if they did. There is a legitimate oral alternative, however: The FDA approved Rybelsus, a semaglutide tablet for type 2 diabetics, in 2019.
International News
Europe’s medicine regulator warned about counterfeit GLP-1s. Pakistani authorities seized expired pharmaceutical ingredients.
The European Medicines Agency warned about an uptick of illegal semaglutide, liraglutide, and tirzepatide marketed and sold via Facebook profiles, advertisements, and e-commerce listings.
Authorities in Punjab, Pakistan intercepted 82 drums and 21 sacks of expired chemicals on their way to pharmaceutical manufacturers.