Posts by psm2016
FDA Alert: UPDATED: Torrent Pharmaceuticals Limited Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP and Losartan Potassium and Hydrochlorothiazide Tablets, USP
The impurity detected in the active ingredient for Losartan is N-nitrosodiethylamine (NDEA), which is a substance that occurs naturally in certain foods, drinking water, air pollution, and industrial processes, and has been classified as a probable human carcinogen as per international Agency for Research on Cancer (IARC) classification. Torrent is only recalling lots of losartan containing products that contain Nitrosodiethylamine (NDEA) above the acceptable daily intake levels released by the FDA.
[...]Partnership for Safe Medicines’ Statement on New Drug Importation Bills
Washington, D.C. (January 10, 2019) – Marv Shepherd, President of the Partnership for Safe Medicines, released the following statement today in response to new legislation, the “Affordable and Safe Prescription Drug Importation Act,” and the “Safe and Affordable Drugs from Canada Act of 2019” which would allow medicines to be imported into the United States…
[...]FDA Alert: Happy Together, Inc. Issues Voluntary Nationwide Recall of Product Due to Presence of Undeclared Sildenafil and Tadalafil
Happy Together, Inc. Boynton Beach, FL is voluntarily recalling all lots within expiry of the Rhino 5k capsules to the consumer level. FDA analysis founds these products to be tainted with sildenafil and Tadalafil. Sildenafil/Tadalafil is an FDA approved drug for the treatment of erectile dysfunction, the presence of sildenafil in the Rhino 5k products renders them unapproved drugs for which safety and efficacy have not been established, therefor subject to recall.
[...]Multi-State Counterfeit Pill Drug Trafficking Organization Halted by 400+ Law Enforcement Officers on December 5th and 6th
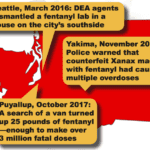
A series of drug seizures by the Bremerton Police Department last year has led Federal authorities to a massive drug trafficking organization that was responsible for bringing thousands of counterfeit oxycodone pills made with fentanyl into Skagit and Snohomish counties.
[...]Seattle Area Drug Trafficker Allegedly Used Pill Press to Make Counterfeit Xanax
Federal drug trafficking charges have been filed against Gizachew Wondie, a resident of the Capitol Hill section of Seattle, on charges that he was manufacturing and selling counterfeit Xanax pills and other drugs. In addition to the discovery of a handgun, investigators found approximately 11,000 ‘Xanax’ pills stored in large plastic bags, and a large pill press.
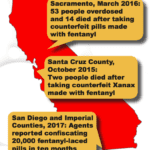
[...]Bay Area Adderall Counterfeiter Sentenced to 10 Years in Prison
A Brisbane, California resident has been sentenced to ten years in prison for manufacturing and distributing counterfeit Adderall, according to a press release from the U.S. Department of Justice (DOJ). Gino Carl von Eckstein, who pleaded guilty in September, was originally charged with producing fake Adderall pills with pill presses located at a house he was using in San Leandro.
[...]FDA warns consumers to avoid Rhino male enhancement products found at retailers because of undeclared and potentially dangerous drug ingredients
The U.S. Food and Drug Administration is warning consumers not to purchase or use Rhino male enhancement products, due to a recent rise in reported health issues. Since 2007, the FDA has identified more than 25 products marketed with variations of the name “Rhino” that contained hidden drug ingredient(s).
[...]CNN Warns that Fake Drugs are a Global Industry That Puts Your Life at Risk
In a Mosaic Science article shared by CNN, author Srinath Perur delves into the global pharmaceutical industry and discovers that in India, counterfeit and substandard medications are an everyday occurrence.
[...]Fake Oxycodone Dealer Indicted in San Diego After a Man Dies from Fentanyl Poisoning
The U.S. Justice Department (DOJ) have announced the arrest and indictment of a 23 year-old Highland, California man accused of selling counterfeit oxycodone pills that killed a La Jolla resident.
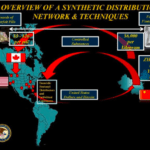
[...]Drug Importation and the Deadly Challenge of Screening 275 Million Packages a Year
The Food and Drug Administration (FDA) in their report U.S. Food and Drug Administration and the International Mail Facilities (IMFs), describes the daunting job that Customs and Border Protection (CBP) faces when attempting to weed out counterfeit medications and packages containing illicit fentanyl. In 2017, IMFs received 275 million packages. Of these, 10,000 were screened by CBP, and of those 86% contained drugs. The investigation of a suspect package is incredibly time-consuming; an experienced FDA investigator might take as long as 20 minutes to process a package containing just on product.
[...]