Posts by PSM Author
April 7, 2025: A study examines FDA-OCI’s enforcement actions over a five-year period
A study examined the 130 enforcement actions undertaken by the U.S. Food and Drug Administration’s Office of Criminal Investigations from 2016 through 2021.
[...]Colorado’s March 2025 Amended SIP Application
The state of Colorado submitted a revised application to the U.S. Food and Drug Administration to operation a Canadian drug importation plan in March. This blog post examines how the state’s application has changed over the years.
[...]FOIA Returns from towns using Alternative Funding Vendors
PSM has sent open records requests to several towns and schools with self-funded health plans about their relationships with Alternative Funding Vendors.
[...]FDA Alert: Update on Previous Nationwide Warning About Presence of Undeclared API in Capsules
The FDA published an update to a previous alert about undeclared active pharmaceutical ingredients in a dietary supplement to expand the package styles affected by this warning.
[...]FDA Alert: Recall Issued on Nasal Wash System Due to Microbial Contamination
An FDA alert shared information on a nationwide recall of a nasal wash system due to microbrial contamination.
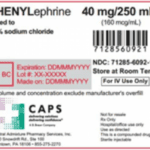
[...]FDA Alert: Recall Issued on Injectable Product Due to Potential Particulate Matter
An FDA alert warned that Central Admixture Pharmacy Services issued a nationwide recall of Phenylephrine 40 mg added to 0.9% Sodium Chloride 250 mL in 250 mL Excel Bag due to the detection of black particulate matter in a single sealed vial of Phenylephrine Hydrochloride.
[...]FDA Alert: Recall Issued on Dietary Supplement Containing Active Pharmaceutical Ingredient
An FDA alert warned that Vitafer-L has been found to contain undeclared tadalafil, an active pharmaceutical ingredient. Products containing tadalafil cannot be marketed as dietary supplements.
[...]December 16, 2024: Black market medicine in Texas and Tennessee
Two new cases involving black market medicine in Texas and Tennessee, and CPB seized thousands of pills in Laredo.
[...]December 9, 2024: Lawsuit against Snap to proceed
The lawsuit against Snap may proceed and the ADA issued a statement about compounded GLP-1 medicines.
[...]JAMA Personal Drug Importation Correction
PSM’s executive director submitted the following correction to the Journal of the American Medical Association (JAMA) in December 2024: International online pharmacies are illegal, and an unacceptable cost-saving strategy Shabbir Safdar Executive Director, The Partnership for Safe Medicines JAMA’s October 21, 2024 Special Communication, “Strategies to Help Patients Navigate High Prescription Drug Costs,” by Hussain…
[...]