Posts Tagged ‘Botox’
FDA alert: Counterfeit version of Botox found in multiple states
The products appear to have been purchased from unlicensed sources. Medications purchased from unlicensed sources may be misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective and/or unsafe.
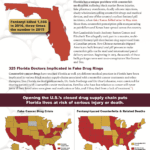
[...]Florida Couple Prosecuted for Selling Fake Botox Wholesale
The recent prosecution of Jorge Nogueira and Jessyka Molina for selling counterfeit Botox wholesale highlights one of Florida’s biggest counterfeit drug problems: fake beauty injections. This is the third action against fake beauty injections in Florida in the last year.
[...]Newly Launched Federal Task Force Finds Fake Xanax and Botox in First Week of Operation at Michigan Ports of Entry
The Detroit branch of the newly launched Global Trade Task Force (GTTF) has found counterfeit medications amongst the $1 million worth of counterfeit goods seized during one week. The seizures occurred at the Detroit Metro Airport, and the Port Huron Blue Water Bridge ports of entry.
[...]Cosmetic Injectable Treatments Now Used to Treat Dozens of Ailments so Fakes Poses A Danger to More U. S. Patients
In 2016 alone, over 1,400 practitioners were warned by the FDA that their supplier was selling unapproved so-called “Botox.” With more than 2,400 professionals warned in the past 5 years, the expansion of use for this medication provides new markets for counterfeiters selling fake and misbranded drugs. Botulinum neurotoxin, or Botox as it is known,…
[...]Real Housewives Botox Nurse Pleads Guilty to Buying Misbranded Botox from Fake Online Pharmacy in Canada
California nurse who provided Botox treatments to the stars of Real Housewives of Orange County has pleaded guilty to charges she treated her patients with non-FDA approved Botox. Her original supplier of the misbranded Botox was SB Medical, an illicit Canadian medication importer that paid $75 million in fines and Penalties in 2015 for illegally…
[...]Neurology Associates of Greenville Sentenced in Misbranded Botox Case
South Carolina medical practice prosecuted for buying non-FDA approved injectable cosmetic treatments. A medical practice in Greenville, South Carolina has been sentenced to 3 years of federal probation for treating patients with non-FDA approved Botox, a Department of Justice (DOJ) press release reported on the occasion of the sentencing. According to the DOJ, “Records obtained…
[...]Counterfeit Beauty Injections Claim the Lives of 2 in Southern Texas
Southern Texas has experienced a rash of injuries and 2 deaths that have resulted from counterfeit cosmetic injections. In 2014, Elva Navarro was prosecuted for administering counterfeit beauty injections. Navarro received a 5-year sentence for the death of one of her clients, and another 3 years for injecting her clients with a fake dermal filler that…
[...]Fake Botox on the Rise in US as FDA Warnings and Recent Arrest Indicate
In 2012, the FDA sent over 350 warning letters to doctors advising them they may have purchased fraudulent or misbranded injectable drugs, including fake versions of Avastin, Botox, and two different osteoporosis treatments. Now the FDA has identified another batch of fake Botox that is currently being marketed to doctor via fax blast.
On April 26, 2013, the US Food and Drug Administration (FDA) posted a drug safety warning for healthcare practitioners, warning them that fraudulent versions of the anti-wrinkle treatment Botox are being marketed and sold in the US. The Botox is being sold by unlicensed suppliers, and has not been vetted within the secure U.S. supply chain. They state that the FDA “cannot confirm that the manufacture, quality, storage, and handling of these products follow U.S. standards. These fraudulent products are considered unsafe and should not be used.“
[...]