News
Counterfeit Injections Can Lead to Disfigurement and Death
A new documentary called “Killer Curves: Bodies to Die For” showed how dangerous, and even deadly, it can be to get silicone butt injections. Sidney Star, K. Michelle, Apryl Michelle Brown, and Anivia Cruz all spoke about their regrets and the damage those shots did to their bodies…
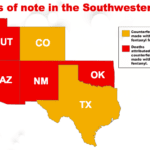
[...]Counterfeit Fentanyl Pills and Powder Being Seized In Ever Larger Amounts In Arizona
Law enforcement in Arizona has seen the amount of seized fentanyl powder increase by 2,000 and the number of fentanyl pills increase by 3,000 in just one year. Despite the increased seizures, the number of lives lost in that state to opioid overdoses also continues to rise. With fake pills being reported across the state – in Kingman, Phoenix, and Tucson, no pill bought on the street can be considered safe…
[...]New York Residents Charged After Being Found With Over 12 Kilos Of Counterfeit Xanax Pills
The year-long Operation Dark Gold operation netted charges against 35 individuals selling drugs online. Included in this list are five people from New York – Jian Qu, Raymond Weng, Kai Wu, Dimitri Tseperkas, and Chiad Akkaya- who sold multiple types of drugs and were found in possession of four pill presses and over 12 kilos of suspected counterfeit Xanax pills…
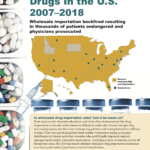
[...]Between 2007 and 2018 Foreign Wholesalers Sold American Doctors Unreliable Black Market Cancer Drugs
From 2007 to 2018 American physicians and clinics demonstrated that drug importation is not safe and is extremely difficult to make safe. Doctors thought they were saving money. Instead they purchased illegally imported, expired, damaged and outright counterfeit medications—including cancer treatments—from black market wholesalers posing as licensed distributors in Canada and other countries.
[...]Consumer Reports Advises Consumers To Shop Around To Find The Best Price
The safest and best place any American can buy their prescription drugs from is a U.S. licensed pharmacy. However, not all pharmacies charge the same amount for the same prescription. You can save money by comparing prices around town…
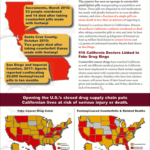
[...]DOJ Indicts Two In Northern California Who Distributed Counterfeit Pills Made With Fentanyl
U.S. Department of Justice announced an indictment against Alfredo Sanchez and Saybyn Borges for distribution and possession with intent to distribute counterfeit oxycodone pills that were made with fentanyl. In just over one month’s time, the DEA says the pair sold over 7,500 fake pills…
[...]New Mexico Authorities Warn Of Increase in Counterfeit Fentanyl Pills
Law enforcement in New Mexico is worried after seeing an increase in the number of counterfeit pills made with fentanyl that are being found. The state lost over 20 people to fake oxycodone pills in 2016 and U.S. Customs and Border Protection keeps seeing the amount of fentanyl they are seizing increase…
[...]Proposed Changes to WHOIS system privacy will help hide internet criminals, NABP director warns
In this August 1, 2018 editorial for Inside Sources, National Association of Boards of Pharmacy Director Carmen Catizone raises the alarm about ICANN’s proposed changes to the WHOIS system, a database that identifies the owners of web domains. These changes are meant to bring WHOIS in compliance with new European privacy laws but, he warns, they would also impede law enforcement and others’ efforts to “connect the dots and link up different websites run as part of large criminal enterprises” like drug counterfeiting rings.
[...]Pill Presses, Punches and Dies Found in Home of Man Indicted for Possession of Counterfeit Pills Made with Methamphetamine
The U.S. Department of Justice indicted Gino Carl von Eckstein after he was found in possession of counterfeit Adderall pills he manufactured using methamphetamine. A search of his car and several properties turned up fake pills, additional powder, and two pills presses needed to make the pills…
[...]Miami-Dade County Woman Indicted For Performing Illegal Silicone Injections
The U.S. Department of Justice indicted a woman in south Florida who was illegally injecting silicone into the bodies of her clients for body contouring purposes. The FDA has never approved silicone injects for this purpose due possible consequences including pulmonary embolism, infection, chest pain, and death…
[...]