About PSM
Prescription Drug Freight Fraud Report, October 2025
What large-scale commercial imports made it into the country between March and August of 2025?
[...]Med spas need stronger regulation
Medical spas and wellness clinics are operating on the edge of existing regulatory frameworks and it’s a risk to American patients.
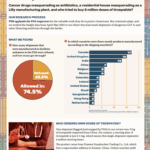
[...]Prescription Drug Freight Fraud Report, September 2025
Our analysis of data from June and July revealed hundreds of pharmaceutical shipments that entered the U.S. from facilities no one would expect, including unregistered Chinese exporters and alternative medicine clinics abroad.
[...]Handout: The fake pill trade hasn’t gone away
Handout: The fake pill trade hasn’t gone away It’s been 10 years since deadly counterfeit prescription pills began killing Americans. News about fake pill deaths has become commonplace and advocates have made progress to protect lives, but many people still don’t know about this threat or the pill presses that make it possible. Share this…
[...]Public health groups raise alarm about fake HIV medicines in New York
PSM and ADAP Advocacy Association filed an official complaint with the State of New York regarding allegations that City Plus Care Pharmacy Inc. dispensed counterfeit HIV medication to a patient in Queens, New York.
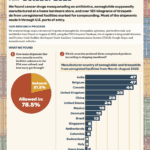
[...]Prescription Drug Freight Fraud Report, July 2025
What if your tirzepatide shipment came from a Brazilian beauty clinic? Or a vial of semaglutide was manufactured, supposedly, at a Costco in Toronto? In April and May 2025, dozens of shipments of semaglutide, tirzepatide, apixaban, and antibiotics entered the U.S. from facilities that aren’t in the FDA’s drug manufacturing database. These aren’t low-volume personal-use…
[...]Partnership for Safe Medicines Applauds Reintroduction of the Cooper Davis Devin Norring Act
The Partnership for Safe Medicines (PSM) today announced its strong support for the reintroduction of the Cooper Davis Devin Norring Act, a bipartisan effort aimed at curbing the online sale of deadly counterfeit and illicit drugs.
[...]Pill press update: January through June 2025
Pill presses and molds are used to make fake pills with deadly consequences thousands of people every year. The number of Americans that take fake pills annually without a fatal event is even higher. Read our January through June 2025 report covering pill press seizures, policy developments, and legislation.
[...]The End of GLP-1 Compounding
Federally-registered compounding facilities stopped making tirzepatide on May 22. This transition is significant for patients, and we at PSM think there are four things you should be watching for.
[...]Empower Pharmacy’s no good, very bad week
Articles in Endpoints News and the Houston Chronicle raise questions about the regulatory process for 503B compounding facilities.
[...]