About PSM
New PSM ads highlight concerns of FDA Commissioner Gottlieb about the dangers of Florida’s importation legislation
Tallahassee, FL (April 18, 2019) – Today the Partnership for Safe Medicines released new ads to run in several parts of Florida that highlight the dangers of Florida attempting to import medicine from Canada. Five different commissioners of the US Food and Drug Administration, appointed by both Republicans and Democrats, have stated that such proposals are dangerous to patients, will expose them to counterfeits, and are unlikely to reduce the price of medicine.
[...]Partnership for Safe Medicines Launches Advertising Campaign in Florida to Oppose Importation
April 12, 2019 (Tallahassee, FL) — Importation undermines our core efforts to keep our medicine supply safe. State and federal authorities regulate every entity in the U.S. supply chain from the point of manufacture until a medicine is dispensed, and that makes counterfeits in the legitimate supply chain rare. In 2013, Congress passed the Drug…
[...]PRESS RELEASE: Over 100 Organizations Representing Law Enforcement Officers, Healthcare Professionals, Patient Advocates Urge White House and Congress to Reject Prescription Drug Importation
WASHINGTON (April 8, 2019) – As members of Congress and leaders in the executive branch consider steps to allow wholesale importation of prescription medications, organizations representing consumers, employers, healthcare professionals, patients, and law enforcement officers, among others, are insisting that such proposals, if implemented, would endanger the health and safety of the communities throughout the country…
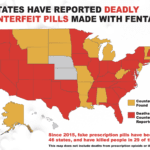
[...]Illegal Pill Presses: An Overlooked Threat to American Patients (2019)
The supply of and demand for dangerous counterfeit and illegally-imported medications has created one of our country’s most serious health challenges—and the prevalence of illegal pill presses are contributing to this crisis. For more information Visit the report launch site Read our 2021 update.
[...]New Report: Broad Availability of Illegal Pill Presses Creates Unprecedented Threat to America’s Communities, Patients, and Law Enforcement
Tallahassee, Florida (March 19, 2019) — The supply and demand of dangerous counterfeit and illegally-imported medications is posing one of our country’s most serious health challenges, and illegal pill presses are directly contributing to this crisis, a new report jointly released today by The National Association of Boards of Pharmacy, National Association of Drug Diversion Investigators, and The Partnership for Safe Medicines has found….
[...]Partnership for Safe Medicines’ Statement on Florida’s Drug Importation Proposals
[UPDATE: This statement has been updated to include all three pieces of pending legislation in Florida. March 5, 2019] Washington, D.C. (March 5, 2019) – Shabbir Safdar, Executive Director of the Partnership for Safe Medicines, released the following statement today in response to three separate proposals from Florida Governor Ron DeSantis and and the state…
[...]In Annual Hill Briefing, Law Enforcement Authorities and Current and Former DEA Agents Share Details on Danger Posed by Counterfeit Drugs and Illicit Fentanyl Imports
In Capitol Hill briefings sponsored by the Partnership for Safe Medicines (PSM) on January 31, former and current law enforcement leaders sounded a warning over the increased flow of counterfeit drugs and lethal fentanyl into the United States, noting that international drug traffickers and crime syndicates see our nation as a lucrative market.
[...]PSM Went to Congress to Share Our Concerns About The Threat of Counterfeit Medicine
On Thursday, January 31, 2018, the Partnership for Safe Medicines held two briefings in Washington, D.C. to inform members of Congress and their staff about the dangers posed to Americans by counterfeit medicines. The events each had three panels and looked at how fake medicines have affected individuals and law enforcement, and also at the roles played by international bad actors and drug cartels…
[...]Partnership For Safe Medicines 2019 Congressional Briefings
On Thursday, January 31, 2019, victims of counterfeit medicines and their families, local law enforcement, former DEA agents, and other experts in the fight against counterfeit medicines met for a discussion about the widespread impact fake drugs are having on communities and on the enormous burden the problem places on regulators who are responsible for our drug safety.
Learn more and watch the briefing here.
[...]Partnership for Safe Medicines’ Statement on New Drug Importation Bills
Washington, D.C. (January 10, 2019) – Marv Shepherd, President of the Partnership for Safe Medicines, released the following statement today in response to new legislation, the “Affordable and Safe Prescription Drug Importation Act,” and the “Safe and Affordable Drugs from Canada Act of 2019” which would allow medicines to be imported into the United States…
[...]