Press Releases
PSM urges the House Energy & Commerce Committee to support the SAFE Drugs Act of 2025
PSM wrote the House Energy & Commerce Committee in support of the Safeguarding Americans from Fraudulent and Experimental (SAFE) Drugs Act, to help protect the American drug supply from unsafely compounded medication.
[...]Public health groups raise alarm about fake HIV medicines in New York
PSM and ADAP Advocacy Association filed an official complaint with the State of New York regarding allegations that City Plus Care Pharmacy Inc. dispensed counterfeit HIV medication to a patient in Queens, New York.
[...]Partnership for Safe Medicines Applauds Reintroduction of the Cooper Davis Devin Norring Act
The Partnership for Safe Medicines (PSM) today announced its strong support for the reintroduction of the Cooper Davis Devin Norring Act, a bipartisan effort aimed at curbing the online sale of deadly counterfeit and illicit drugs.
[...]PSM submits comment on CBP’s proposed rules to improve screening of low-value shipments
On March 14, the Partnership for Safe Medicines submitted comment on Customs and Border Protection’s proposed update to regulations around low-value, “de minimus” shipments.
[...]New report reveals illegal ingredients for knockoff weight loss drugs flooding into U.S. from foreign sources, endangering patient safety
The Partnership for Safe Medicines today released a new report that found suspicious, unauthorized, and illegal ingredients for popular diabetes and obesity injectables (commonly known as weight loss drugs) are flooding into the U.S. from foreign sources despite U.S. laws forbidding them from coming through the border.
[...]Partnership for Safe Medicines statement on weight loss drug ad blitz
Partnership for Safe Medicines released the following statement in response to the advertisement that lifestyle brand Hims & Hers released ahead of the Super Bowl to sell unregulated compounded weight loss drugs
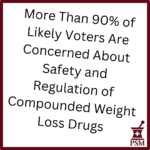
[...]PSM Compounded Weight Loss Drugs Poll
PSM commissioned a survey about compounded diabetes and weight loss injectable drugs. 1,000 likely voters across political affiliations were crystal clear — they are worried about safety and the current lack of oversight. See the full results.
[...]PSM applauds the reintroduction of the SHOP SAFE Act
On June 13, 2024, PSM sent a letter to Congressmen Darrell Issa and Jerrold Nadler applauding the reintroduction of the Stopping Harmful Offers on Platforms by Screening Against Fake E-Commerce (SHOP SAFE) Act.
[...]The Partnership for Safe Medicines supports pharmacy benefit reforms in California Senate Bill 966
State Senator Scott Wiener’s bill would eliminate under-reimbursement practices that create a dangerous opportunity for criminals to enter the legitimate supply chain.
[...]PSM Advisory Board approves policy resolution on reimbursement practices and supply chain safety
PSM opposes the practice of reimbursing pharmacies below the cost of acquisition of medicine and supports efforts to reform reimbursement systems that eliminate this market dynamic that threatens the safety of our drug supply.
[...]