PSM Publications
Prescription Drug Freight Fraud Report, July 2025
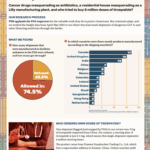
What if your tirzepatide shipment came from a Brazilian beauty clinic? Or a vial of semaglutide was manufactured, supposedly, at a Costco in Toronto? In April and May 2025, dozens of shipments of semaglutide, tirzepatide, apixaban, and antibiotics entered the U.S. from facilities that aren’t in the FDA’s drug manufacturing database. These aren’t low-volume personal-use…
[...]Pill press update: January through June 2025
Pill presses and molds are used to make fake pills with deadly consequences thousands of people every year. The number of Americans that take fake pills annually without a fatal event is even higher. Read our January through June 2025 report covering pill press seizures, policy developments, and legislation.
[...]Prescription Drug Freight Fraud Report, May 2025
What if your blood thinners were made in an unverified location in Colombia? In March 2025, dozens of pharmaceutical shipments entered the U.S. that were manufactured in places no one would expect.
[...]Handout: Illegal ingredients linked to knockoff weight loss drugs pouring in from foreign sources
Compounded versions of GLP-1 injectable treatments for diabetes and obesity have surged in popularity despite a lack the safety and efficacy assurances. The FDA has warned that these knockoff versions sometimes contain illicit semaglutide or tirzepatide—the active pharmaceutical ingredients (APIs) in weight loss drugs. Working with George Karavetsos, former director of FDA’s Office of Criminal…
[...]Prescription drug affordability boards by the numbers
Prescription drug affordability boards by the numbers (March 2025) Since prescription drug affordability boards (PDABS) were first started in 2020 in Maryland, they’ve been copied in Colorado, Minnesota, Oregon, Washington, and New Jersey. Their goal has been to lower the price of medicine, but they haven’t yet saved any money. Download and share our infographic…
[...]Prescription Drug Affordability Boards by the numbers
Prescription Drug Affordability Boards are an expensive idea that has not produced results. It’s time to move on.
[...]Handout: What is an Alternative Funding Program?
Some employers are hiring consultants, sometimes called “Alternative Funding Program (AFP) vendors” or
“importation program vendors,” to supply employees and their families with illegally imported and unsafe
medicines.
New report reveals illegal ingredients for knockoff weight loss drugs flooding into U.S. from foreign sources, endangering patient safety
The Partnership for Safe Medicines today released a new report that found suspicious, unauthorized, and illegal ingredients for popular diabetes and obesity injectables (commonly known as weight loss drugs) are flooding into the U.S. from foreign sources despite U.S. laws forbidding them from coming through the border.
[...]Five years of PDABs: broken promises, rising costs, and risks to access
Five years of PDABs: broken promises, rising costs, and risks to access (January 2025) Prescription Drug Affordability Boards (PDABs) have been created in several states with the goal to lower prescription drug costs for patients. One of the oldest, Maryland’s, is five years old, but it has yet to fulfill its promise to lower medicine…
[...]Patients face safety risks with counterfeit and compounded prescription weight-loss drugs
Officials are concerned about compounded weight-loss medicines as demand surges. Share this PDF to spread the news
[...]