Article Topic
Are below cost reimbursement practices by Pharmacy Benefit Managers creating opportunity for criminals to enter the legitimate supply chain?
PBMs, by under reimbursing pharmacies, are creating a demand for pharmacies to seek lower priced medications even when they can’t exist at that price.. Criminals appear to be happy to become part of the supply chain.
[...]Has any state tried Canadian drug importation? How did it work for them?
The state of Maine opened it’s border to Canadian drug importation program for Maine residents. Dr. Kenneth McCall purchased and tested some of the medicines being advertised and sold to Maine residents and found them lacking in safety. Listen to his story.
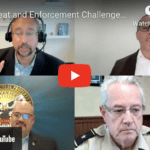
[...]The threat and enforcement challenges of counterfeit medicines
PSM’s Shabbir Imber Safdar sat down with three law enforcement experts about the problems they are seeing with counterfeit medicine. Watch here.
[...]Are Canadian patients struggling with drug shortages already?
PSM’s Shabbir Imber Safdar speaks to Canadian patient advocate Durhane Wong-Rieger about the drug shortage situation in Canada.
[...]What medicines do U.S. states want to import from Canada’s drug supply?
What drugs have states said they plan to export from Canada’s drug supply? We combed all their applications to the FDA and compiled that list into one document.
[...]January 29, 2024: Family advocates distribute lifesaving medicine at Naloxone Hill Day in Washington DC
Family advocates Andrea Thomas and Amy Neville will distribute naloxone at the Dirksen Senate Office Building. The VA will use the NABP’s digital DSCSA platform to help with compliance. Guilty pleas for dark web drug trafficking and manufacturing fake oxycodone, and more.
[...]Plea Agreement, USA v Farace, October 2018
United States District Court District of Maryland USA v Ryan Farace Plea Agreement Filed October 3, 2018 Read the document.
[...]Judgment, USA v Farace, January 2024
United States District Court District of Maryland USA v Ryan and Joseph Farace Judgment Filed January 6, 2024 Read the document.
[...]Plea Agreement, USA v Farace, September 2022
United States District Court District of Maryland USA v Ryan and Joseph Farace Plea Agreement Filed September 8, 2022 Read the document.
[...]The Partnership for Safe Medicines supports S. 2943, the SHOP SAFE Act
On January 17, 2024 PSM wrote the Senate Subcommittee on Intellectual Property to endorse S.2934, which would expand the Trademark Act of 1946 to extend liability for selling harmful counterfeits to online platforms. Read the letter here.
[...]