Article Topic
NABP RogueRx Activity Report examines illegal sales of injectable weight loss drugs
The NABP reports that illegal actors are selling desperate patients substandard and counterfeit versions of the new generation of weight loss medicine.
[...]FDA Alert: Florida Company’s Dietary Supplement Recalled for Containing Prescription Drug Ingredients
STOP CLOPEZ CORP is voluntarily recalling one lot of Schwinnng capsules to the consumer level. FDA analysis has found the Schwinnng products to contain Nortadalafil. Nortadalafil is an active drug ingredient known for the treatment of male erectile dysfunction. The presence of Nortadalafil in Schwinnng capsules makes it an unapproved new drug for which the safety and efficacy have not been established and, therefore subject to recall.
[...]April 22, 2024: NY federal courts close cases involving fraud, second-hand HIV meds
The Southern District of New York sentenced criminals from two separate HIV drug diversion rings.
[...]Indictment, USA v Sukhjit Singh Ghuman et al, September 2023
United States District Court Southern District of California USA v Sukhjit Singh Ghuman, et al Indictment Filed September 29, 2023 Read the document.
[...]Superseding Indictment, USA v Sukhjit Singh Ghuman et al, October 2023
United States District Court Southern District of California USA v Sukhjit Singh Ghuman, et al Superseding Indictment Filed October 13, 2023 Read the document.
[...]Government’s Sentencing Memo, USA v Ryan Stabile, February 2024
United States District Court District of Massachusetts USA v Ryan Stabile Government’s Sentencing Memo Filed February 9, 2024 Read the document.
[...]Indictment, USA v Ryan Stabile, October 2019
United States District Court District of Massachusetts USA v Ryan Stabile Indictment Filed October 24, 2019 Read the document.
[...]Defendant’s Sentencing Memo, USA v Ryan Stabile, February 2024
United States District Court District of Massachusetts USA v Ryan Stabile Defendant’s Sentencing Memo Filed February 13, 2024 Read the document.
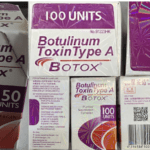
[...]FDA alert: Counterfeit version of Botox found in multiple states
The products appear to have been purchased from unlicensed sources. Medications purchased from unlicensed sources may be misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective and/or unsafe.
[...]PSM asks major online pharmacy sales platforms for information
PSM asks major online pharmacy sales platforms for information Today, the Partnership for Safe Medicines wrote to the leading online platforms that enable pharmacy-to-pharmacy drug sales to address a potential danger to the safety of the drug supply. PSM is concerned that counterfeit and diverted medication might be sold on online pharmacy-to-pharmacy platforms by criminals…
[...]