Article Topic
Counterfeit Pills Still Being Found Despite DEA Successes In Seizing Precursor Ingredients
Three news stories recently out of Arizona show that despite the DEA taking over 120,000 counterfeit fentanyl pills off the streets in 2017, police keep finding more and seizures of a precursor chemical needed to produce fentanyl are up…
[...]Shoreside Enterprises Issues Voluntary Nationwide Recall of 7K and Poseidon 4500 (Extreme 1000 Mg) Due to Presence of Undeclared Sildenafil and Tadalafil
This is a reprint of an FDA Alert. When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does not endorse either the product or the company. For Immediate Release May 17, 2018 Contact Consumers Shoreside Enterprises (727) 236-0576 Shoreside Enterprises, Inc. is…
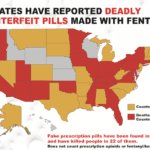
[...]Counterfeit Fentanyl Pills Harming Iowa Residents
The U.S. Attorney’s Office for the Northern District of Iowa issued a warning to residents about counterfeit pills made with fentanyl being sold on the streets around the state. Shortly after this announcement, the DOJ announced guilty pleas from two Waverly residents charged with selling counterfeit pills made with carfentanil and cyclopropyl fentanyl that they purchased off the Internet…
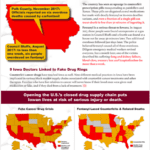
[...]Wholesale Drug Importation From Canada Won’t Lower Prices, But Will Increase Health Risks Says Vermonter
By approving the wholesale reimportation of U.S. prescription drugs from Canada, the Vermont Legislature passed an illegal measure that will not lower drug prices. Instead, it will subject Vermonters to public health risks and new taxes to defray an inevitable federal lawsuit.
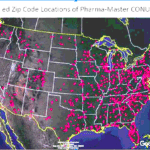
[...]More Charges in Utah-Based Counterfeit Pills Case
An additional individual has been charged for his role in the Pharma-Master counterfeit fentanyl pill ring allegedly run by Utah-resident Aaron Shamo. Jonathan Luke Paz received four charges in federal court…
[...]Research into Fake Online Pharmacy Prosecutions Finds Current Policies Do Little to Stop Them
A 2017 legal review conducted by Camille Guerra and Dr. Timothy K. Mackey predates the CanadaDrugs prosecution, however it clearly illustrates the challenges faced by courts in effectively prosecuting illicit online pharmacies
[...]Center for Safe Internet Pharmacies and Partnership for Drug-Free Kids Announce New Coalition
The Center for Safe Internet Pharmacies and Partnership for Drug-Free Kids launched MedicineSafe to educate families about medicine safety.
[...]Fentanyl Pill Counterfeiters In Arkansas, California, and North Carolina Busted
Stories of counterfeit pills made with fentanyl keep coming in from across the country. PSM did a quick recap of three stories – one from Arkansas, one from North Carolina, and one from California – that we want to make sure you know about…
[...]Illicitly Manufactured Fentanyl Poisonings Doubled Nationwide Between 2015 and 2016
Drug overdose deaths increased 27.9% since 2015, and the CDC notes, “These increases primarily were driven by deaths involving synthetic opioids, for which the rate doubled from 2015 to 2016.” Synthetic opioids like illicit manufactured fentanyl are poisoning Americans in record numbers.
[...]Jury Convicts Florida Doctor For Making Counterfeit Pills With Fentanyl
A federal jury found Dr. Johnny C. Benjamin of Vero Beach guilty on five of the seven charges against him, including manufacturing counterfeit pills with fentanyl that caused the death of a young woman in Wellington, Florida…
[...]