A clandestine fake drug factory in Colombia washes empty medication bottles before filling them with counterfeit Tazocin, a prescription antibiotic. Photo courtesy of Pfizer. On February 10th, 2017, the National Association of Boards of Pharmacies wrote a letter to the U.S. Congress about the risks associated with Canadian online pharmacies. The letter, below in text…
Read MoreJuan Gallinal, a former Virginia police officer, acted as the head of a Florida conspiracy that used a valid brick-and-mortar pharmacy as a cover for series of fake pharmacy websites that sold misbranded and addictive drugs. The owner of a fake online pharmacy business in Florida has been sentenced to eight years in prison, the…
Read MoreThe Justice Department, the United States Food and Drug Administration (FDA) and the Drug Enforcement Administration (DEA) are concerned about Americans illegally purchasing prescription medicines from foreign sources. According to News Channel 10, investigations are underway to protect Americans from these potentially dangerous substances. The Justice Department has been looking at the data and as…
Read MoreArizona resident Betty Hunter died of lung cancer, but how much longer would she have lived if her oncologist had not treated her with counterfeit Avastin? A recent Danish documentary tells the tragic story of Arizona grandmother Betty Hunter, who in 2011 sought treatment for her lung cancer at an oncology clinic in Chandler Arizona.…
Read MoreAn investigation into the corruption of former Governor Javier Duarte has uncovered allegations that children with cancer were treated in state-run hospitals in Veracruz with counterfeit chemotherapy medicines due to budget shortfalls and lack of resources caused by his administration. The BBC reports, that when tested, the counterfeit chemotherapy drugs came back as an inert…
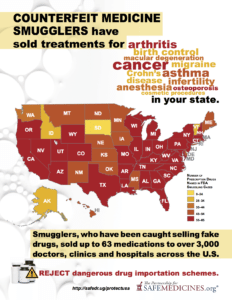
Read MoreSince 2012, the FDA has issued warnings to more than 3,000 doctors, clinics and hospitals about eight different breaches in the U.S. drug supply chain by rogue distributors such as Richards Pharma, Canada Drugs, Medical Device King, Gallant Pharmaceuticals, and TC Medical. These rogue distributors offered 63 different non-FDA approved medicines. Have you or has someone you know taken medicine that these smugglers have sold?
Read MoreThe two men acted as U.S. distributors for a series of Thailand-based fake online pharmacy websites that sold imported and non-FDA approved medication to U.S. consumers. Troy Tapia and Anthony Rouse III of Louisiana have each been sentenced to three years probation after pleading guilty to fraud conspiracy, wire fraud, mail fraud and money laundering,…
Read MoreIn 2016 alone, over 1,400 practitioners were warned by the FDA that their supplier was selling unapproved so-called “Botox.” With more than 2,400 professionals warned in the past 5 years, the expansion of use for this medication provides new markets for counterfeiters selling fake and misbranded drugs. Botulinum neurotoxin, or Botox as it is known,…
Read MoreJanuary 5, 2017 Dear Senators and Representatives, In December of 2015 we released the attached letter warning Congress of the dangers to American patients from counterfeit drugs brought into America through “importation” proposals. As you consider the budget this year, we wanted to bring to your attention several recent tragic events that show the negative…
Read MoreDefendants allegedly purchased expired or close-to-expired gastric lap-band surgery kits on the Internet, disguised them as new, and then re-sold them to local physicians. Florida residents Peter Kakfa and Gregory Grimm are being accused of running an expired/misbranded gastric bypass banding system scam via their employer Apollo Endosurgery, the Department of Justice (DOJ) reports. According…
Read More