Search:
Filter:
Sort:
A July 1, 2025 post clarifies that applicants can use “a static baseline approach for the cost-savings analysis” instead of trying to account for changes in unpredictable markets.
Read MoreThe operation launched 1,728 investigations in 91 countries and shut down 13,000 criminal-linked websites, social media pages, channels, and bots used to market and sell illegal or counterfeit medicines.
Read MoreUnited States District CourtSouthern District of Florida USA v Adam Brosius Plea Agreement Filed April 2025 Read the document.
Read MoreOn June 26, PSM commented in support proposed revisions to forms FDA 3500 and FDA 3500B, which are used to report adverse events from prescription and over-the-counter medicines.
Read MoreMore than 40 cases of botulism linked to Botox injections have been reported in the U.S. and U.K. over the last two months.
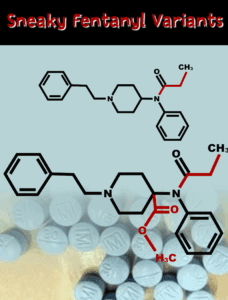
Read MoreThe bill permanently schedules fentanyl analogues as Schedule I drugs under the Controlled Substances Act.
Read MoreThe Jha brothers admitted selling counterfeit Keytruda and other prescription drugs to undercover agents.
Read MorePSM is soliciting feedback on draft best practices until June 20th. Please review the guidelines and send your thoughts to editors@safemedicines.org.
Read MoreUnited States District Court Western District of Washington USA v Rajnish Kumar Jha Plea Agreement Filed June 2025 Read the document.
Read MoreUnited States District Court Western District of Washington USA v Avanish Kumar Jha Plea Agreement Filed June 2025 Read the document.
Read More