Proposals to import medicine from Canada can't work. And when they fail, they will endanger all Americans.
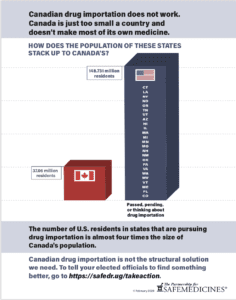
Canada is too small a country to act as the U.S.'s pharmacy. Click to learn more.
What: HHS has proposed draft federal regulations to import medication in bulk from Canada. Comments can be made until March 9, 2020. The American Pharmacist Association, the American Society of Health System Pharmacists, the National Association of Boards of Pharmacy, and the National Association of Chain Drug Stores have announced their opposition to drug importation.
How: Using the form at right to tell HHS why this idea doesn't work, and suggest alternate ways of bringing down health care costs.
What to say: You should use your own words, but here are some examples:
- Americans need real action to lower their costs at the pharmacy counter. Importing drugs from Canada won’t work, and people will suffer when Canada refuses to hand over their drug supply. Stop this idea and work on real solutions to address patients’ costs.
- America has nine times more people than Canada. It’s impossible for Canada to fulfill Americans’ medicine supply. What’s worse? Counterfeiters can step in, putting citizens from both countries at risk. Stop pretending with plans like Canadian importation and find a better way to deal with high costs at the pharmacy.
- This plan doesn’t make any sense. The Canadians have already said they don’t want anything to do with it. Stop wasting time and enact real change to bring down patients’ drug costs like capping out of pocket costs in Medicare Part D.
- Americans want real solutions to medication costs, not gimmicky rule changes that can never be enacted since Canada has no interest in playing along. What's going to happen when Canada prevents us from taking their drugs? This is a terrible idea. Find a workable solution that helps American patients instead.
More ideas can be found in PSM's one-pager on the draft HHS rules [PDF] and our comments filed with HHS.