How to Stay Safe as a Patient
Patients are growing concerned about reports of counterfeit drugs being provided by doctors. Patients can protect themselves by asking their physicians questions about the origins of medicines they receive in their doctor’s office.
Read more about what patients can do to stay safe.
By Tom Kubic, President and CEO of the Pharmaceutical Security Institute
Patients are growing concerned about reports of counterfeit drugs being provided by doctors. In February, 19 U.S. cancer clinics were warned by the FDA that they had received counterfeit versions of a cancer drug. Patients can protect themselves by asking their physicians questions about the origins of medicines they receive in their doctor’s office.
Authentic Avastin™ is made in the U.S. and moves through a closed, secure drug supply chain to American cancer clinics. The counterfeit medication, however, crossed four continents before arriving in the U.S. via unauthorized distributors. Investigators are working to identify those responsible for manufacturing this fake medicine. One thing they do know is that the fake product was not the vital, life saving chemotherapy patients expected. The fakes contained none of the therapeutic ingredients.
How could a patient have known that the medication was fake? The chemotherapy drug is a clear liquid, so no patient with an IV in his arm would have known what ingredients were in the IV. However a cursory look at the box the medications came in would have immediately revealed peculiarities that should have raised alarms. Even if the patient had never seen the box before, the French and Arabic lettering on the package was a dead give-away that something was wrong!
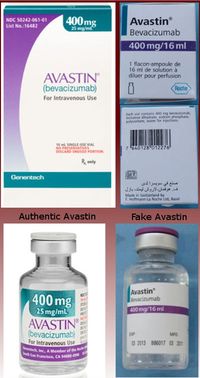
Additionally, the package falsely states that the product was made in Switzerland, when the authentic product is made in the US. Also, the packaging itself, the color, design and typeface used on both the box, and the interior bottle are significantly different from the authentic product. Lastly, the manufacturer is incorrectly identified, instead of saying “Genentech, a member of the Roche Group” it says “Made in Switzerland by F. Hoffman-La Roche Ltd.”[1] If the patients would ask to see the medication upon every visit, the fake packaging would have alerted them to a problem with this medicine.
Sadly this is not the only US incident of counterfeit medications being foisted upon patients at doctor’s offices.
Respiratory paralysis, near death, and disfigurement have been the unfortunate results of fake Botox treatments in the U.S in the past several years[2][3][4]. The rise in popularity of the treatment has coincided with the importation of dangerous and deadly counterfeits. Recently in the US, Chinese Botox fakes were found with concentrations differing by 500% than what was stated on label and that used materials that can cause severe allergic reactions.
In December 2010, a San Diego doctor pleaded guilty to injecting patients with supposed neuropeptides to cure cancer, arthritis and other chronic conditions. However the patients were actually injected with glucocorticoid steroids that caused severe bone density loss. Additionally he sold an undercover FBI agent so called dietary supplements for pancreatic cancer treatment which contained nimesulide, a medication unapproved by the FDA and removed from the European and Asian markets because of high rates of liver failure.
Patients can protect themselves by being curious about their medications. Dr. Bryan Liang, Executive Director and E. Donald Shapiro Distinguished Professor of Health Law, at the Institute of Health Law Studies at California Western School of Law, exhorts, “First, if the price is too good to be true, walk away. Second, examine the bottle being used in your treatment. Ensure that it hasn’t been used, isn’t expired and that it’s labeled correctly.[5]” Protect yourself by writing down the identifying information on the medication the doctor’s office is giving you, including the serial and lot numbers.
Doctors, nurses, pharmacists and medical office workers can all keep an eye open for foreign drugs in the inventory at their work place. Whether the medication arrives inadvertently due to supplier error, or due to deceitful practices, anyone can report the foreign medications to the U.S. Food and Drug Administration’s Office of Criminal Investigations.
Call them at 800-551-3989.
Or visit the OCI’s website (www.accessdata.fda.gov/scripts/email/oc/oci/contact.cfm).
Or email them at DrugSupplyChainIntegrity@fda.hhs.gov.
If you want your doctor, nurse or pharmacist to learn more about protecting your medications and the closed, secured drug supply chain, download and give them copy of the Partnership for Safe Medicine’s LEADERs Guide.