PSM Releases Report on Black Market IUDs at U.S. Clinics
 Download and share the Black Market IUD Cases Resource which highlights important cases of illegally imported, non-FDA approved IUDs that were purchased by doctors and inserted into unsuspecting patients at clinics in the U.S. in the past five years.
Download and share the Black Market IUD Cases Resource which highlights important cases of illegally imported, non-FDA approved IUDs that were purchased by doctors and inserted into unsuspecting patients at clinics in the U.S. in the past five years.
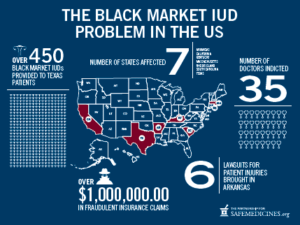
Since 2009, 35 doctors in 7 states have been prosecuted for exposing women to non-FDA approved IUDs. Doctors have been accused of purchasing IUDs from “Canadian” fake online pharmacies and importing untested IUDs from Mexico, then billing Medicare for the cost of genuine, FDA-approved IUDs.
It is estimated that over 450 women have been implanted with misbranded, black-market IUDs since 2009. In 2010, the FDA issued a public warning to all consumers concerning the dangers posed by black market IUDs. In the letter, Teresa Togio, FDA’s liaison with health professionals stated, “The recent issue with patients in Rhode Island unknowingly receiving imported, unapproved IUD/IUSs highlights the unacceptable risk patients may be exposed to when a product’s identity, purity, source, handling, and storage cannot be verified.”
To learn more about the epidemic of doctors purchasing misbranded & non-FDA approved IUDs, Download BlackMarketIUD_singlepage5.pdf
Court Documents related to Black Market IUDs
United States v Scully, et al. (Medical Device King)
- Sentencing Memo, September 12, 2018 (William Scully)
- Guilty Plea, May 24, 2018 (William Scully)
- Opinion, U.S. Court of Appeals, December 23, 2017
- Memorandum of Decision and Order, 3/16/2016
- Superseding Indictment, July 22, 2015
- Transcript of Criminal Cause for Pleading, October 16, 2014 (Shahrad Rodi Lameh)
United States v Paul Singh
- Order Imposing Restitution, July 26, 2016
- Memorandum of Plea Agreement, July 31, 2015
United States v James S. Buck
- Information, April 1, 2013
- Plea Agreement, May 9, 2013
- Sentencing, August 12, 2013
United States v Cahn Jeff Vo
- Indictment, March 20, 2013
United States v Bayardo Cruz
- Decision of the Medical Board of California, April 14, 2011