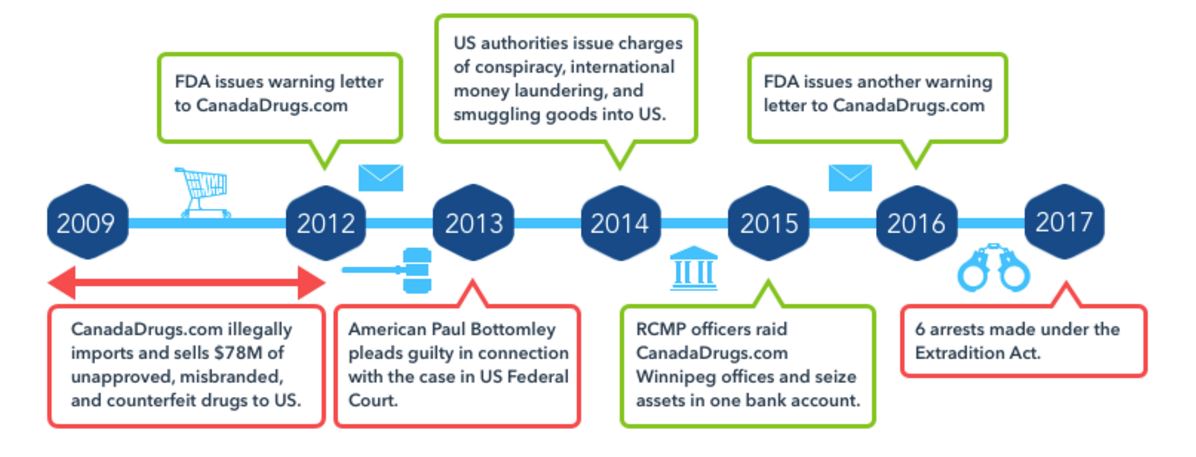
CanadaDrugs Case Affidavit Reveals Disregard for Patient Safety
According to CBC News, new information has come to light in the case against six executives of CanadaDrugs.com. The company, its affiliates, and associates in the United Kingdom and Barbados stand accused of illegally importing and selling $78 million worth of unapproved, misbranded and counterfeit drugs to American doctors between 2009 and 2012. The court documents show new details, including the June 6, 2017 affidavit by Royal Canadian Mounted Police (RCMP) Constable Michael Menard detailing the surveillance by officers at five Winnipeg homes from May 15 - 17.
The surveillance of the accused continued despite statements in the affidavit from the lawyers of their clients’ willingness to turn themselves in, having already surrendered their passports to counsel or being will to do so. The affidavit also reveals email conversations between the accused. Some of these correspondents came from a March 2015 raid of CanadaDrugs.com’s office. According to the affidavit, the emails between Sigurdson, Chalus, Nakamura, and Trueman were authenticated by Microsoft.
New information also included emails between James Trueman, a liaison for CanadaDrugs.com, and employees at International Market Access, Inc. (IMA). IMA was hired to receive CanadaDrugs.com’s foreign-sourced prescription drugs, keep the inventory in their warehouse and ship to physicians in the U.S. In one email, IMA personnel notified Trueman that a physician had returned two units of a drug because the cold-chain drug was not received cold. Trueman's response was, “Just put in freezer they will be ok.” In another email, Trueman directed IMA personnel to make a note of which doctors were returning products because the freezer packs were warm by the time the drugs arrived. He said, “...next time use a couple of bigger freezer pacs from the US and we will see if they still complain.” Prosecutors allege bacteria contaminated some drugs sold by CanadaDrugs.com because of improper handling.
The affidavit referenced a conversation taped by U.S. investigators in December 2011 between an employee at a cancer clinic and a U.S. affiliate of CanadaDrugs.com. When the employee of the cancer center asked if the drugs being sold were approved by the U.S. Food and Drug Administration (FDA), the answer from an employee of Montana Healthcare Solutions was, “No, the drugs we sold were never FDA approved… That is why you are able to get the prices you are getting.”
Witnesses in the upcoming trial are expected to include scientists, employees of cancer clinics and special agents from the FDA. Paul Bottomley, Brian Phillips and Ram Kamath, associates of Thorkelson’s, are also expected to testify as well.