Oregon Doctor Admits To Injecting Patients With Non FDA-Approved Anti-Wrinkle Treatments
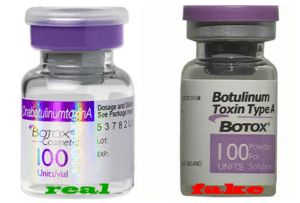
Source: The Counterfeit Report
The Oregonian reported that Brenda Roberts, a 54-year-old physician, pleaded guilty in June to a misdemeanor after she admitted to injecting patients with non U.S. Food and Drug Administration (FDA)-approved wrinkle-reducing drugs that she purchased from rogue online pharmacies. Roberts made purchases from www.unitedpharmacies.com, a non-licensed online pharmacy that LegitScript has identified as rogue. A rogue pharmacy may prescribe controlled substances without requiring a prescription, is one that does not adhere to accepted pharmacy practices, or engages in fraudulent or deceptive business practices. When the U.S. Drug Enforcement Agency (DEA) and the FDA began their investigations, Brenda Roberts voluntarily surrendered her license to practice medicine.
The DEA’s investigation found that Roberts was also operating a rogue online pharmacy out of her home. The Oregonian referenced a search warrant affidavit that stated that Roberts distributed controlled substances to customers throughout the U.S., although she admitted to purchasing drugs like lorazepam, temazepam, hydrocodone, and tramadol allegedly to administer to patients after injecting them with the non FDA-approved wrinkle reducing drugs.
Roberts purchased legitimate Botox until 2008 when her account was shut down for violating their terms of service. Assistant U.S. Attorney Donna Maddux said it was at this time that Roberts turned to purchasing drugs from overseas websites and that she would administer the drugs at “Botox parties she had in her home.” In November 2016, investigators obtained an empty Botox vial from Roberts’ garbage that was not manufactured for use in the U.S. An April 2017 search of her home turned up files that indicated she was injecting patients there.
The maximum sentence for the offense is one year, a $100,000 fine and a year of supervised release, but prosecutors will be recommending that Roberts be sentenced to six months of probation. In a separate civil case, the U.S. Attorney’s Office alleges that Roberts purchased and distributed phentermine, a drug that is often prescribed for weight loss. The government expects her to face a fine in that case.