New Mexico Pharmacists Association Expresses Concern Over Allowing Drug Importation In Letter to Congress
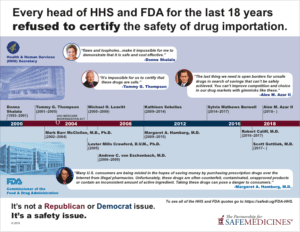
The rising cost of prescription drugs has been a concern for many Americans for years, and some legislators have long seen allowing drug importation as a potential remedy for their constituents. However, consistent concerns about safety have led every head of the U.S. Department of Health and Human Services and the Food and Drug Administration (FDA) for the past 19 years to say that drug importation is not feasible. Despite these two agencies, whose missions are the protection of the health of all Americans, being against allowing drug importation, legislators continue to float the idea.
In response, organizations send letters to members of Congress expressing their concerns over the possible legalization of drug importation. In March 2019, PSM sent a letter signed by over 100 advocacy organizations to the White House and Congress warning them that allowing the importation of non-FDA-approved medications would endanger the health and safety of communities across the country. One of the organizations that signed onto that letter, the New Mexico Pharmacists Association (NMPhA), had so many concerns that they just sent a letter to members of Congress.
NMPhA cited seven reasons for their concerns ranging across a variety of topics such as U.S. federal drug safety policy, Canadian law, consumer buying habits, and the difficulty of holding international bad actors responsible. Additionally, NMPhA noted that allowing the importation of drugs would complicate, if not undermine, the implementation of the Drug Supply Chain Security Act (DSCSA). The DSCSA established the track and trace protocols for all prescription pharmaceuticals to prevent counterfeit and unapproved medications from getting into the hands of U.S. patients. Track and trace is not scheduled to be fully implemented until 2023.
PSM had the opportunity to speak with NMPhA Executive Director Dale Tinker about the realities and misconceptions that people have about prescription drugs and drug importation. When asked for the truth about drug importation that he feels the average American citizen does not understand, Tinker said, “Our FDA carefully reviews medications to make sure they are safe and effective. Other countries don’t do that, including Canada when the drugs are for export to the U.S.” Surrounding the confusion that people have about where their medications come from, Tinker posited that “people generally don’t think about drug approval by the FDA as being a safety issue, but more of a burdensome government involvement.” Asked if he would let his family members purchase medicine from a non-FDA-approved foreign source, Tinker said, “I would not. The safety of my family is more important than saving a few dollars – or even a lot of dollars.”
Being against drug importation is not a Republican or Democrat issue. It is a safety issue.