What if you were a cancer patient and the only thing protecting you from being treated with counterfeit medication was an especially vigilant team of oncology nurses?
United States vs. McLeod Cancer and Blood Center
In 2007, McLeod Cancer and Blood Center in Johnson City, Tennessee received a fax from a company called Quality Specialty Products (QSP)—also known as Montana Healthcare Solutions—that promised them lower prices on chemotherapy drugs. Knowing that lower-priced medicines would be good for their bottom line McLeod changed their suppliers, even though QSP was selling medicine acquired from foreign sources that had not been inspected or approved by the FDA.
Because the medicine QSP was sending was not labelled in English, McLeod’s oncology nurses realized immediately that it could not be FDA-approved. According to court documents, they confronted the practice’s doctors and office manager and refused to give these drugs to their vulnerable patients. Practice manager Dr. William Kincaid and office manager Michael Dean Combs agreed not to buy black market medicine from QSP any more.
In 2009, however, a sales representative from QSP met with Kincaid and Combs at a local restaurant and persuaded them to resume their business relationship. Kincaid directed his office manager to have QSP’s black market medicines shipped to a storage facility and concealed the purchases from nursing staff by mixing them in with the clinic’s legitimate drug supply.
Between September 2009 and February 2012, Kincaid and Combs bought $2 million in illegally imported drugs, and made a profit of $500,000 by billing Medicare and other government benefits programs as if they had paid the full price for FDA-approved drugs.
Ultimately, Michael Dean Combs and William Kincaid pleaded guilty for their roles in purchasing illegal foreign drugs. Kincaid was sentenced to two years in prison and to pay $2.55 million to settle civil claims in 2013. Combs got three years of probation.
What does “FDA-approved” mean?
Since 1962, the U.S. government has required the Food and Drug Administration (FDA) to establish that medicines sold to U.S. patients are safe and effective for the conditions which they claim to treat. Only about 12% of drugs in development make it through this process, which takes an average of 12 years of clinical testing by pharmaceutical companies per drug. When they do, pharmaceutical companies submit a New Drug Application (NDA) during which the FDA reviews the research and testing, approves a drug’s professional labelling, and inspects the facilities where it will be manufactured. If the agency is satisfied, it will approve the drug for sale in the U.S.
After approval, FDA conducts surveillance inspections of both domestic and foreign manufacturing facilities to make certain that they meet U.S quality standards, and additional “for cause” inspections if they have reason to be concerned about manufacturing safety.
Why are non-FDA approved medicines a problem?
Developed countries all over the world have processes to regulate medicine so that their citizens can generally trust that it is safe and effective. They do not, however, regulate medicine sold to patients in the U.S. That’s the FDA’s role.
An unlicensed wholesaler selling illegally imported medicines has sidestepped regulatory systems in both their country of origin and in the United States. The FDA has no idea where the products they sell were manufactured or under what conditions, whether they are made of the correct ingredients, or whether they have been adulterated or damaged in transit. Unsurprisingly, regulators have encountered many counterfeit and substandard imported medicines in the last 20 years.
When you order imported medicine online, it is impossible to be sure what you are getting.
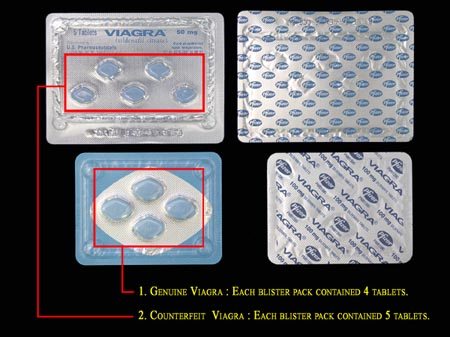
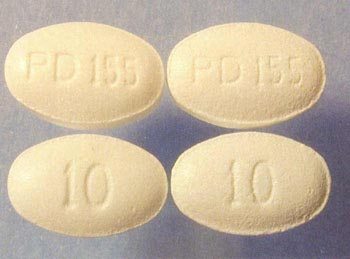
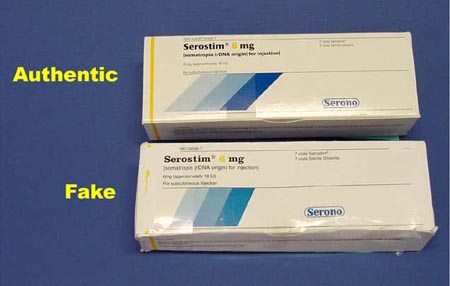

Examples of counterfeits found by the FDA.
Counterfeit Cancer Medication
Unfortunately, McLeod Cancer and Blood Center was not alone in purchasing unapproved medication from overseas. Companies like QSP sent fax blasts to doctors’ offices across the country, and many medical practices felt lower prices were worth the risks.
In 2012 and 2013, however, three different counterfeits of a cancer drug called Avastin reached U.S. medical practices. The medicine came from QSP, the same wholesaler that had sold McLeod Cancer and Blood Center black market products. This time, QSP, which was purchased by a division of Canada Drugs in 2010, had sold Avastin that had no active ingredient.
The FDA sent letters to more than 900 offices in 48 states in 2012 and 2013, warning that they might have purchased medicines from sellers who had distributed counterfeit Avastin. Over the next four years FDA warned more than 3,000 doctors’ offices to stop buying a broad variety of infused and injected medicines from sketchy foreign distributors.
It took six years for the diligent agents at the FDA’s Office of Criminal Investigation, and U.S. Attorney’s offices in Missouri, New York and Montana to prosecute executives at six companies involved in counterfeit cancer drug supply chains, including:
- Ozay Pharmaceuticals
- Richards Pharma
- Medical Device King
- Montana Healthcare Solutions
- Ban Dune Marketing
- Canada Drugs and subsidiaries.
Doctors, medical practices and black market drug sellers were prosecuted in 17 states. Those convicted were sentenced to serve a cumulative 107 years of prison time and probation and to pay roughly $84 million in fines and restitution.
 You can read more about this ugly era in counterfeit drug history in our report, Black Market Cancer Drugs in the U.S.
You can read more about this ugly era in counterfeit drug history in our report, Black Market Cancer Drugs in the U.S.
If you or a loved one was treated by one of these doctors, we’d like to hear your story.
Please check these lists of medical practices that the FDA warned for buying black market drugs and contact us at editors@safemedicines.org.
QSP sold unapproved cancer and osteoporosis medicines, as well as Botox. FDA letters were sent: Nov 2012 • Sept 2012 • June 2012 • Apr 23, 2012 • Apr 5, 2012 • Feb 2012
Medical Device King sold 26 different unapproved medicines, including a counterfeit cancer drug. The FDA sent doctors letters in May 2013.
Gallant Pharma sold 39 different unapproved medicines. The FDA warned doctors in April 2015.
TC Medical sold counterfeit Botox and 21 other unapproved medicines. The FDA sent letters on Mar 30, 2016 and Mar 21, 2016.
Sources for this week's video and blog
- “August 23, 2012: California Man Sentenced for Importing Adulterated Cancer Drugs; Forfeits $1.4 Million & Land Rover Automobile,” U.S. Department of Justice press release, U.S. FDA, August 23, 2012.
- Black Market Cancer Drugs In the U.S. 2007–2018, The Partnership for Safe Medicines.
- Becky Campbell, “Johnson City Cancer Center Manager Gets Probation for Role in Mislabeled Drugs Case, Johnson City Press, April 1, 2013.
- “Counterfeit Lipitor Found in Legitimate Pharmaceutical Supply Chain,” The Pharmaceutical Journal, August 5, 2005.
- “FDA Identifies Third Case of Fake Cancer Drug Distributed in the US,” Fox News, February 6, 2013.
- “FDA Issues Letters to Doctors Who May Have Purchased Counterfeit or Unapproved Prescription Drugs,” U.S. FDA, last updated July 3, 2019.
- Kristina Fiore, “Viagra from Online Sources Mostly Fake,” MedPage Today, August 30, 2012.
- John Glionna, “Bootleg Pharmaceuticals Flood Immigrant Communities Cut Off From Healthcare,” The Los Angeles Times, September 18, 2019.
- “Historical Information: FDA Issues Letters to Doctors Who May Have Purchased Counterfeit or Unapproved Prescription Drugs, 2012-2014,” U.S. FDA, last updated July 2, 2019.
- “Johnson City Physician Sentenced to Serve Two Years in Prison for Violations Related to Unapproved Foreign Drugs,” U.S. District Court of the Eastern District of Tennessee press release, Federal Bureau of Investigations, June 11, 2013.
- “July 11, 2013: English Citizen Sentenced for Distributing Adulterated and Counterfeit Cancer Drugs,” U.S. Department of Justice press release, U.S. FDA, July 11, 2013.
- “July 12, 2013: Paul Daniel Bottomley Sentenced in U.S. District Court,” U.S. Department of Justice press release, U.S. FDA, July 12, 2013.
- Medical Practices That May Have Received Prescription Drugs From Pharmalogical, Inc., D/B/A Medical Device King, U.S. FDA, May 13, 2013
- Medical Practices That Purchased Multiple Medications From Clinical Care, QSP, Montana Healthcare Solutions, or Bridgewater Medical, U.S. FDA, June 28, 2012, April 23, 2012, April 5, 2012.
- Medical Practices That Purchased Medication From Quality Special Products (QSP), Also Known as Montana Health Care Solutions, U.S. FDA, February 10, 2012.
- “Medical Device King Exec Takes a Plea Deal Instead of a Retrial; Gets 32 Months in Prison,” Partnership for Safe Medicines, July 17, 2019.
- “Myth: We Are Getting the Same Drugs Canadians Take,” The Partnership for Safe Medicines.
- “Online Pharmacy Sentenced for Illegal Imports,” The Associated Press, April 13, 2018.
- Karen Pauls, “Canadian Links Sought in U.S. Counterfeit Drug Probe,” CBC News, June 20, 2012.
- Bernard Simon, “Canada Rebuffs U.S. on Prescription Drugs,” The New York Times, November 19, 2003.
- Plea Agreement, USA v William Ralph Kincaid, U.S. District Court of the Eastern District of Tennessee, Case: 2:12-cr-00116, November 15, 2012.
- “Second Turkish Man Sentenced for Smuggling Counterfeit Cancer Drugs,” FDA, January 23, 2015.
- “Securing the U.S. Drug Supply Chain: Oversight of FDA’s Foreign Inspection Program,” Testimony of Janet Woodcock, M.D., Director of the Center for Drug Evaluation and Research to the House Committee on Energy and Commerce, Subcommittee on Oversight and Investigations, November 10, 2019.
- Gail A.Van Norman, “Drugs, Devices, and the FDA: Part 1: An Overview of Approval Processes for Drugs,” JACC: Basic to Translational Science, April 2016.
- Warning letter to Doctors Who Purchased Unapproved Drugs or Devices From T.C. Medical, U.S. FDA, undated.
- Christopher Weaver, “U.S. Fake-Drug Probe Puts Spotlight on Role of Doctors,” The Wall Street Journal, November 20, 2012.
- --- “Former Internet Pharmacist Sentenced in Fake Drug Case,” The Wall Street Journal, January 9, 2013.

