Medical Device King Exec Takes a Plea Deal Instead of a Retrial; Gets 32 Months in Prison
Between 2009 and 2013 Great Neck, New York-based Pharmalogical, Inc. grossed $17 million selling black market imported medicine, among them cancer treatments, Intrauterine Devices (IUDs), and Botox, to U.S. medical practices who believed they were buying FDA-approved products. The company’s president, William “Liam” Scully, was found guilty of these activities in 2015, but the Second Circuit Court of Appeals vacated the conviction in 2017. Last year, he was re-sentenced to 32 months in prison.
According to court documents, Scully founded Pharmalogical with a colleague, Shahrad Rodi Lameh. They began selling imported Botox and Mirena IUDs under the name Medical Device King (MDK) in 2009 and expanded into the oncology market in late-2010 or early-2011. On May 24, 2012, FDA agents searched MDK’s offices while hunting for the source of counterfeit versions of the cancer drug Avastin. MDK stopped selling oncology drugs after the FDA visit, but Scully continued oncology sales through a new company, Taranis Medical Group, without Lameh’s knowledge.
On May 13, 2013, the FDA warned almost 800 medical clinics that they may have purchased counterfeit versions of Altuzan (a Turkish medicine that contains the same active ingredient as Avastin) from MDK in late 2011. MDK purchased products from Ozay Pharmaceuticals, which had supplied Richards Pharma with counterfeit versions of the Altuzan, some of which, upon testing, had no active ingredient.
In April 2014, the Justice Department indicted Scully and Lameh on 73 counts of conspiracy, mail fraud, wire fraud, distribution of misbranded and counterfeit prescription drugs, trafficking in counterfeit goods, and smuggling. In October 2014, Lameh pleaded guilty to conspiracy to commit wire fraud and conspiracy to distribute misbranded drugs. He received a sentence of three years probation and agreed to pay a $500,000 money judgment.
Scully went to trial where, the Justice Department reported, 40 witnesses testified that he deceived doctors into believing that he was selling FDA-approved medicines rather than “unapproved products imported through a series of unidentified middlemen in Turkey and elsewhere.” On November 12, 2015, a jury found Scully guilty of 64 felonies. He was sentenced to five years in prison, and to pay $900,000 in forfeitures.
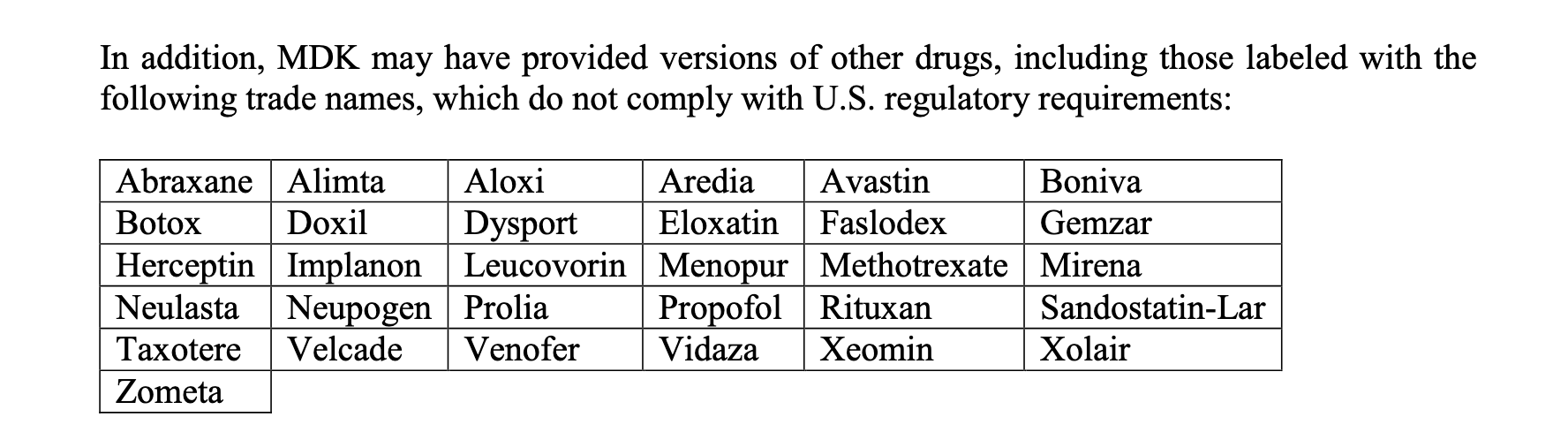
non-FDA approved Aclasta and MabThera, and these 31
additional products.
The U.S. Court of Appeals for the Second Circuit vacated Scully's conviction in December 2017 because the lower court had excluded evidence showing he sought legal advice about importing drugs with foreign labels. After he pleaded guilty to one felony count of introducing a misbranded drug into interstate commerce in May 2018, Scully was sentenced to 32 months in prison (17 months after time served) the following October.
Scully’s case is one of a series of cases in which wholesalers have imported large volumes of unsafe medicines from unregulated sources and sold them to U.S. medical practices without regard for the health of American patients. Learn more about the dangers of supply chain breaks like these.