Hot issues
Pill presses and molds are used to make fake pills with deadly consequences thousands of people every year. The number of Americans that take fake pills annually without a fatal event is even higher. Read our January through June 2025 report covering pill press seizures, policy developments, and legislation.
Avanish Kumar Jha and Rajnish Kumar Jha got 30-month sentences for selling counterfeit medicines—including vials of fake Keytruda—to U.S. buyers.
After a November 2024 inspection, the FDA issued a Form 483 to Empower Clinic Services highlighting violations related to compounded drug manufacturing practices at its East Windsor, New Jersey facility.
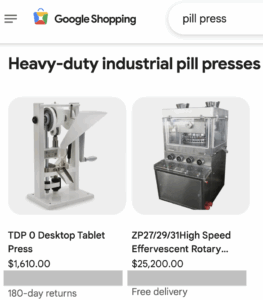
The change will affect the Google Ads and Google Shopping platforms starting in September 2025.
A July 1, 2025 post clarifies that applicants can use “a static baseline approach for the cost-savings analysis” instead of trying to account for changes in unpredictable markets.
The operation launched 1,728 investigations in 91 countries and shut down 13,000 criminal-linked websites, social media pages, channels, and bots used to market and sell illegal or counterfeit medicines.
On June 26, PSM commented in support proposed revisions to forms FDA 3500 and FDA 3500B, which are used to report adverse events from prescription and over-the-counter medicines.
Our podcast covers the latest in pharma crime and medicine safety.
Like your information on video? Subscribe to our YouTube playlist!

Who's investigating a company selling research-grade weight loss injections? Find out..

Updates on two federal prosecutions.

Click the images below to see more recent videos.