Posts Tagged ‘doctor prosecution’
Doctor Sentenced To 40 Months For Distributing Unapproved Medicine
Dr. George Patino convicted and sentenced for conspiracy, distributing Human Growth Hormone (HGH) for unauthorized medical purposes, and smuggling. He brought non-FDA approved HGH over from Mexico to treat patients with it for unapproved medical purposes…
[...]Oncologist Who Bought Cancer Drugs from Fake Drug Sellers Sentenced to Six Years in Prison
Dr. Diana Anda Norbergs, a Florida oncologist convicted in November 2016 of importing misbranded, non-FDA approved cancer drugs from unlicensed suppliers, has been sentenced to almost six years in federal prison, according to a report in the Tampa Bay Times. The Department of Justice (DOJ) indictment alleged that Norbergs purchased prescription cancer treatments from unlicensed foreign…
[...]Naturopathic Doctor Pleads Guilty to Using Misbranded Drug on Patients
A Port Angeles, WA area naturopathic physician pleaded guilty in federal court to one count of introducing misbranded drugs into interstate commerce. Richard Marschall prescribed human chorionic gonadotropin (HCG) to approximately 60 people for weight loss between February 2014 and February 2017.
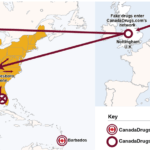
[...]Doctors Who Purchased from CanadaDrugs.com Must Pay Millions in Restitution
Dr. Kenneth D. Nahum, his oncology practice, and Ann Walsh, his wife and the practice manager, have agreed to pay the U.S. $1.7 million to resolve allegations of violating the False Claims Act. They stood accused of ordering drugs from a foreign distributor not approved to sell them in the U.S. and administering those drugs…
[...]Iowa Oncologist Pays $176,460 Fine in Illegally Imported Cancer Drugs Case
Dr. Magdy Elsawy has admitted to no wrongdoing in his settlement, however it was originally alleged he provided non-FDA approved cancer medications to his patients, defrauded Medicare, and provided treatments to patients that were more complex than needed or entirely medically unneeded. North Iowa Today is reporting that the owner and lead physician at Hematology…
[...]Florida Oncologist Convicted for Buying Misbranded Cancer Drugs from Supplier of Fake Avastin
Dr. Diana Anda Norbergs was convicted by a Federal Grand Jury on 45 counts related to her multi-year practice of treating her oncology patients with imported, non-FDA approved cancer medications, and then billing Medicare, public, and private insurers for the full cost of the genuine treatments. The U.S. Department of Justice (DOJ) is reporting that…
[...]Tehachapi Gynecologist Spent 6 Months in Jail For Implanting Women with Non-FDA Approved IUDs
Dr. Paul S. Singh is currently serving one year of home detention after pleading guilty to charges that he deceived his patients and defrauded Medicare by implanting his patients with non-FDA approved IUDs purchased on the Internet. Earlier this year, a Southern California gynecologist, Dr. Paul S. Singh was sentenced to 6 months in prison,…
[...]Neurology Associates of Greenville Sentenced in Misbranded Botox Case
South Carolina medical practice prosecuted for buying non-FDA approved injectable cosmetic treatments. A medical practice in Greenville, South Carolina has been sentenced to 3 years of federal probation for treating patients with non-FDA approved Botox, a Department of Justice (DOJ) press release reported on the occasion of the sentencing. According to the DOJ, “Records obtained…
[...]Connecticut Senator Calls for State Medical Board Intervention in Cases Where Doctors Bought Misbranded Drugs from Gallant Pharma
Although 5 doctors in Connecticut and 256 doctors nationwide have received FDA warning letters concerning purchases of non-FDA approved medication from the now-defunct medicine wholesaler Gallant Pharma International, no state medical boards have investigated any of their practices. Now Senator Richard Blumenthal of Connecticut is calling for the FDA to provide these names to state…
[...]St. Louis Doctor Gets Probation for Treating Patients with Drugs Bought from Fake Online Pharmacy
Dr. Mimlitz specialized in treating men who complained of a lack of energy. His treatments were actually unapproved drugs from South Korea purchased from Mexico via Internet sources. A Saint Louis medical doctor, Dr. Michael Mimlitz, has pleaded guilty to charges of introducing misbranded drugs into Interstate commerce, according to his waiver of indictment. According…
[...]