Posts Tagged ‘FDA’
Canadians Concerned About US Prescription Drug Importation Proposals
John Adams of the Best Medicines Coalition, a Canadian group that represents 28 different patient organizations, has described the Trump administration’s plan to import medication from Canada as a “clear and present danger” to the Canadian prescription drug system, according to AP.
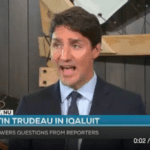
[...]Prime Minister Trudeau Promises to Protect the Canadian Drug Supply In The Face Of The U.S. Drug Importation Statement
Canadian Prime Minister Justin Trudeau was interviewed today in Iqaluit, and according to CBC Canada, he reassured Canadians that Health Canada is already safeguarding the Canadian prescription drug supply regardless of international pressure.
[...]New Analysis by HDA Finds Importation Proposals Are Too Expensive and Pose Health Risks to Patients
A report just released the HDA Research Foundation demonstrates that federal importation proposals to allow drug importation are devoid of practical implementation guidance and funding, so will fail to guarantee federal safety and quality standards for prescription medication.
[...]U.S. FDA Issued Warning Letters About Misbranded Mifepristone And Misoprostol
In March 2019, the U.S. Food and Drug Administration issued warning letters to a website and an online pharmacy network for selling misbranded mifepristone and misoprostol
[...]Millions Of Pills Sold As Herbal Supplements Contained An Active Pharmaceutical Ingredient
Three individuals and four companies have pending plea agreements in federal court on charges related to the illegal importation and sale of $11 million worth of herbal remedies that contained an undisclosed active pharmaceutical ingredient. Over several years, two of the individuals involved manufactured millions of pills…
[...]Keeping The Drug Supply Chain Safe Keeps Patients Safe
In a statement from the U.S. Food and Drug Administration, Commissioner Scott Gottlieb, M.D. discussed the agency’s continuing efforts to combat the spread of illicit opioids and to ensure the security of America’s drug supply chain. Included in their recent efforts, Commissioner Gottlieb noted that the agency has stepped up enforcement and interdiction work at International Mail Facilities and shut down websites illegally selling potentially dangerous, unapproved, and misbranded versions of opioid medications to U.S. citizens…
[...]Doctor Robs Woman of Sight with Unapproved Stem Cell Treatment
Hoping to save her failing vision, a retired woman underwent what turned out to be a non-FDA approved treatment at a stem cell clinic in Georgia in September 2016. By December of that year, both of her retinas had detached, leaving her permanently blinded…
[...]Drug Importation and the Deadly Challenge of Screening 275 Million Packages a Year
The Food and Drug Administration (FDA) in their report U.S. Food and Drug Administration and the International Mail Facilities (IMFs), describes the daunting job that Customs and Border Protection (CBP) faces when attempting to weed out counterfeit medications and packages containing illicit fentanyl. In 2017, IMFs received 275 million packages. Of these, 10,000 were screened by CBP, and of those 86% contained drugs. The investigation of a suspect package is incredibly time-consuming; an experienced FDA investigator might take as long as 20 minutes to process a package containing just on product.
[...]US FDA Joins Interpol and Other International Enforcement Agencies for Operation Pangea XI
The United States Food and Drug Administration (FDA) has announced the conclusion of Operation Pangea XI’s International Week of Action. In its eleventh year, Operation Pangea targets websites that sell counterfeit and misbranded drugs. This year’s effort shut down 465 websites that were selling non-FDA approved prescription drugs including drugs for oncology, antivirals, opioids and other untested prescription drugs to U.S. consumers.
[...]Fat Burners Zone Issues Voluntary Nationwide Recall of Zero Xtreme Due to Presence of Undeclared Sibutramine
Fat Burners Zone is voluntarily recalling 1 lot of Zero Xtreme, capsules to the consumer level. FDA analysis has found Zero Xtreme to be tainted with sibutramine. Sibutramine is an appetite suppressant that was withdrawn from the U.S. market due to safety concerns.
[...]