Posts Tagged ‘FDA’
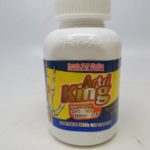
FDA Alert: Herbal Arthritis Treatments Contain Dangerous Prescription Drugs
FDA has received adverse event reports, including of liver toxicity and death, associated with the use of Artri King products, since the agency issued its first warning about an Artri Ajo King product on January 5, 2022.
[...]FDA Issues Warning About Unauthorized Covid-19 At-Home Tests
The U.S. Food and Drug Administration (FDA) is warning people not to use the SD Biosensor Inc. STANDARD Q COVID-19 Ag Home Test. The test is not authorized, cleared, or approved by the FDA for distribution or use in the United States. The FDA is concerned about the risk of false results when using this unauthorized test. This unauthorized test may be packaged in a white and magenta box.
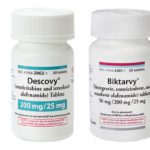
[...]Counterfeit HIV Drug Alert: Gilead Warns of Fake Versions of Biktarvy and Descovy Sold to U.S. Pharmacies
Gilead has alerted potentially impacted pharmacies to investigate the potential for counterfeit or tampered Gilead medication sold by distributors not authorized by Gilead that may be within their recent supply and to remain vigilant to the potential for this to occur in the future. The authenticity and safety of Gilead-branded medicines can only be secure when obtained directly through Gilead’s authorized distributors.
[...]FDA Alert: Food and Drug Administration Issues a More Serious Warning About Methanol in Hand Sanitizers
The FDA has issued a second, more serious warning about hand sanitizers that have been made with deadly methanol. Their first warning came on July 2, when they warned they had “seen a sharp increase in hand sanitizer products that are labeled to contain ethanol (also known as ethyl alcohol) but that have tested positive for methanol contamination.”
[...]Statement on Trump Administration Executive Order on Drug Importation
Washington, D.C. (July 24, 2020) – Shabbir Safdar, executive director of the Partnership for Safe Medicines, released the following statement in response to President Trump’s executive order signed today on the importation of prescription drugs:
[...]4 Deaths and 26 Hospitalizations in Arizona Caused by Fake Hand Sanitizer Made with Methanol
The FDA has issued 70 recalls/warnings at last count for hand sanitizers made with deadly wood alcohol (methanol). Substantial methanol exposure can result in nausea, vomiting, headache, blurred vision, permanent blindness, seizures, coma, permanent damage to the nervous system or death.
[...]FDA Alert: MasterPharm, LLC. Issues Voluntary Nationwide Recall of Finasteride Plus 1.25mg Due to the Presence of an Undeclared Antihypertensive Drug
This is a reprint of an FDA Alert. When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does not endorse either the product or the company. May 11, 2020 Company Contact Information Consumers: Lin Leung, MasterPharm, LLC Phone: (866) 630-5600 Email: recall@masterpharm.com…
[...]Former FDA Associate Commissioner Warns of the “Massive Safety Risks of Importation”
Source: Twitter This editorial by Peter J. Pitts was published in The Times Weekly on March 3, 2020. Mr. Pitts is president of the Center for Medicine in the Public Interest and a former FDA associate commissioner. Keep Canadian drugs out of U.S. medicine cabinets The Trump administration recently proposed two rules that would allow…
[...]FDA Worked With Indian Government To Seize 500 Counterfeit Drug Shipments in January
The U.S. Food and Drug Administration (FDA) has just announced a successful joint operation with the Government of India targeting counterfeit prescription drugs, counterfeit over-the-counter medications, fake medical devices, and misbranded dietary supplements containing harmful ingredients.
[...]Website Operators Warned by FDA/DEA Offered Antibiotics, Cancer & HIV Treatments in Addition to Misbranded Opioids
On September 30th, the U.S. Food and Drug Administration (FDA) and the Drug Enforcement Administration (DEA) issued a joint warning to four online networks that were operating a total of ten fake online pharmacy websites. While all four networks were offering misbranded/counterfeit opioid medications such as tramadol and Soma for sale, three of the online networks had marketplaces offering misbranded medications to treat a kaleidoscope of ailments, such as allergies, cancer, smoking, asthma, and infection.
[...]