Posts Tagged ‘FDA’
PSM leads letter in support of an FDA mandate to destroy high-risk imports
PSM led a coalition of organizations urging leaders of the Senate HELP and House Energy and Commerce committees to help strengthen the FDA’s ability to protect Americans from unsafe and counterfeit medicines and medical products.
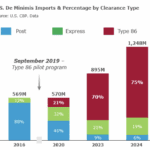
[...]July 21, 2025: Inspection policy for all FDA-regulated products changed at ports of entry
A policy change announced by U.S. Customs and Border Protection means that all FDA-regulated products are now subject to inspection at ports of entry.
[...]An FDA inspection revealed significant problems at Empower’s new outsourcing facility
After a November 2024 inspection, the FDA issued a Form 483 to Empower Clinic Services highlighting violations related to compounded drug manufacturing practices at its East Windsor, New Jersey facility.
[...]FDA announces changes to section 804 importation program proposals
A July 1, 2025 post clarifies that applicants can use “a static baseline approach for the cost-savings analysis” instead of trying to account for changes in unpredictable markets.
[...]PSM writes to support changes to FDA’s Medwatch reporting system
On June 26, PSM commented in support proposed revisions to forms FDA 3500 and FDA 3500B, which are used to report adverse events from prescription and over-the-counter medicines.
[...]The End of GLP-1 Compounding
Federally-registered compounding facilities stopped making tirzepatide on May 22. This transition is significant for patients, and we at PSM think there are four things you should be watching for.
[...]Empower Pharmacy’s no good, very bad week
Articles in Endpoints News and the Houston Chronicle raise questions about the regulatory process for 503B compounding facilities.
[...]FDA Alert: Dietary Supplement Sold on Amazon Recalled for Presence of Prescription Drug Ingredients
The product was distributed via the internet and fulfilled by Amazon nationwide in the USA. On December 17, 2020, FDA issued a press release that warned consumers to avoid certain products found on Amazon, eBay and other retailers due to hidden and potentially dangerous drug ingredients. It also encouraged online marketplaces to ensure these products are not sold on their platforms.
[...]FDA Alert: Class 1 Recall of Some Mesa Biotech COVID Tests Due to Risk of False Positives/Contamination
This is a reprint of an FDA Alert. Mesa Biotech, Inc., Recalls Certain Accula SARS-CoV-2 Tests for Risk of False Positives Caused by Contamination The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death. Recalled Product Product Name: Accula…
[...]FDA Alert: Class 1 Recall on Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test
This is a reprint of an FDA Alert. Do Not Use Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test: FDA Safety Communication May 10, 2022 The U.S. Food and Drug Administration (FDA) is warning people not to use the Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test (Colloidal Gold). This test is not authorized, cleared, or approved…
[...]