The state of Colorado submitted a revised application to the U.S. Food and Drug Administration to operation a Canadian drug importation plan in March. This blog post examines how the state’s application has changed over the years.
Read MoreA March 12, 2025 memo said the state had paid more than $150,000 defending itself in a lawsuit Amgen had filed over plans to set an upper payment limit on its rheumatoid arthritis treatment, Enbrel.
Read MoreSenator Jim Banks asked pointed questions about how FDA will stem the tide of semaglutide and tirzepatide coming from unknown facilities.
Read More“Alternative funding programs” are shifting expensive prescription coverage to illegal drug importation schemes.
Read MoreOn March 14, the Partnership for Safe Medicines submitted comment on Customs and Border Protection’s proposed update to regulations around low-value, “de minimus” shipments.
Read MoreA Texas man billed government programs millions for creams compounded by untrained teenagers. News about counterfeit medicine in six U.S. states.
Read MoreFDA’s shutdown of GLP-1 compounding could lead to a new era of risk for U.S. patients.
Read MoreThe FDA published an update to a previous alert about undeclared active pharmaceutical ingredients in a dietary supplement to expand the package styles affected by this warning.
Read MoreAn FDA alert shared information on a nationwide recall of a nasal wash system due to microbrial contamination.
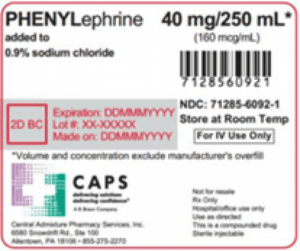
Read MoreAn FDA alert warned that Central Admixture Pharmacy Services issued a nationwide recall of Phenylephrine 40 mg added to 0.9% Sodium Chloride 250 mL in 250 mL Excel Bag due to the detection of black particulate matter in a single sealed vial of Phenylephrine Hydrochloride.
Read More