Avanish Kumar Jha and Rajnish Kumar Jha got 30-month sentences for selling counterfeit medicines—including vials of fake Keytruda—to U.S. buyers.
After a November 2024 inspection, the FDA issued a Form 483 to Empower Clinic Services highlighting violations related to compounded drug manufacturing practices at its East Windsor, New Jersey facility.
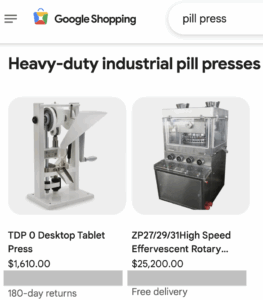
The change will affect the Google Ads and Google Shopping platforms starting in September 2025.
A July 1, 2025 post clarifies that applicants can use “a static baseline approach for the cost-savings analysis” instead of trying to account for changes in unpredictable markets.
The operation launched 1,728 investigations in 91 countries and shut down 13,000 criminal-linked websites, social media pages, channels, and bots used to market and sell illegal or counterfeit medicines.
On June 26, PSM commented in support proposed revisions to forms FDA 3500 and FDA 3500B, which are used to report adverse events from prescription and over-the-counter medicines.
More than 40 cases of botulism linked to Botox injections have been reported in the U.S. and U.K. over the last two months.
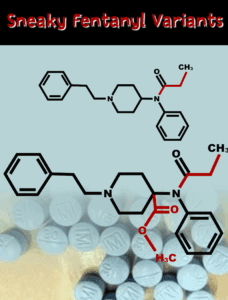
The bill permanently schedules fentanyl analogues as Schedule I drugs under the Controlled Substances Act.
The Jha brothers admitted selling counterfeit Keytruda and other prescription drugs to undercover agents.
PSM is soliciting feedback on draft best practices until June 20th. Please review the guidelines and send your thoughts to editors@safemedicines.org.