Statistics in the agency’s Report on the State of Pharmaceutical Quality show that active pharmaceutical ingredient (API) sites that solely supply compounding pharmacies are disproportionately high risk.
The proposed updates aim to help address safety concerns created by pharmacies reselling compounded sterile drugs purchased from outsourcing facilities.
The Pennsylvania Attorney General filed charges against a pharmacy owner and the Ohio Board of Pharmacy revoked licenses to protect patients.
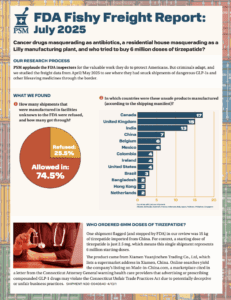
The newest Fishy Freight report continued to find suspect API shipped into the U.S. while a report from the United Nations’ Office of Drug Crime highlighted the dangers of contaminated and counterfeit medicines made with diethylene glycol and ethylene glycol.
What if your tirzepatide shipment came from a Brazilian beauty clinic? Or a vial of semaglutide was manufactured, supposedly, at a Costco in Toronto? In April and May 2025, dozens of shipments of semaglutide, tirzepatide, apixaban, and antibiotics entered the U.S. from facilities that aren’t in the FDA’s drug manufacturing database. These aren’t low-volume personal-use…
We’ve published the final document of best practices for online pharmacy-to-pharmacy marketplaces.
The Partnership for Safe Medicines (PSM) today announced its strong support for the reintroduction of the Cooper Davis Devin Norring Act, a bipartisan effort aimed at curbing the online sale of deadly counterfeit and illicit drugs.
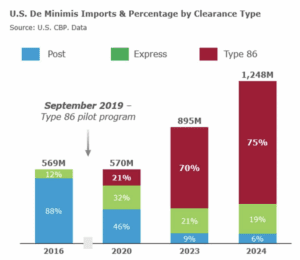
A policy change announced by U.S. Customs and Border Protection means that all FDA-regulated products are now subject to inspection at ports of entry.
503B outsourcing facilities make our drug supply more resilient, but 81% of 503Bs newly registered since June 2021 have never been inspected by FDA staff. Learn why we recommend changes to this important program.
Pill presses and molds are used to make fake pills with deadly consequences thousands of people every year. The number of Americans that take fake pills annually without a fatal event is even higher. Read our January through June 2025 report covering pill press seizures, policy developments, and legislation.