Search:
Filter:
Sort:
503B outsourcing facilities make our drug supply more resilient, but 81% of 503Bs newly registered since June 2021 have never been inspected by FDA staff. Learn why we recommend changes to this important program.
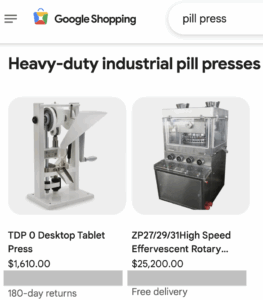
Read MorePill presses and molds are used to make fake pills with deadly consequences thousands of people every year. The number of Americans that take fake pills annually without a fatal event is even higher. Read our January through June 2025 report covering pill press seizures, policy developments, and legislation.
Read MorePrescription Drug Affordability Board Activity, June 2025 Activities Summary Colorado: Colorado’s next PDAB meeting will be held on August 22, 2025. Maryland: Maryland’s PDAB met on June 23 for updates about federal drug pricing and Maryland’s biotech and life sciences industry. Oregon: At its June 18 meeting, Oregon’s PDAB reviewed survey data and narrowed its list of drugs for affordability…
Read MoreAvanish Kumar Jha and Rajnish Kumar Jha got 30-month sentences for selling counterfeit medicines—including vials of fake Keytruda—to U.S. buyers.
Read MoreAfter a November 2024 inspection, the FDA issued a Form 483 to Empower Clinic Services highlighting violations related to compounded drug manufacturing practices at its East Windsor, New Jersey facility.
Read MoreUnited States District Court Western District of Washington USA v Avanish Kumar Jha and Rajnish Kumar Jha Indictment Filed July 2025 Read the document.
Read MoreUnited States District Court Western District of Washington USA v Avanish Kumar Jha Defense sentencing memo Filed July 2025 Read the document.
Read MoreUnited States District Court Western District of Washington USA v Rajnish Kumar Jha Defense sentencing memo Filed July 2025 Read the document.
Read MoreThe change will affect the Google Ads and Google Shopping platforms starting in September 2025.
Read MoreA July 1, 2025 post clarifies that applicants can use “a static baseline approach for the cost-savings analysis” instead of trying to account for changes in unpredictable markets.
Read More