Search:
Filter:
Sort:
Board member Dr. Kenneth McCall was named an associate editor of the Journal of Pharmacy and Pharmaceutical Sciences; Executive Director Shabbir Safdar joined the editorial board of the Journal of Illicit Trade, Financial Crime, and Compliance.
Read MoreA study says IV hydration spas need more oversight. Louisiana residents were hospitalized after receiving black market botulinum toxin injections. Hulu delved into the counterfeit GLP-1 market and Fierce Healthcare covered AFPs.
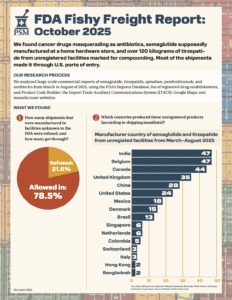
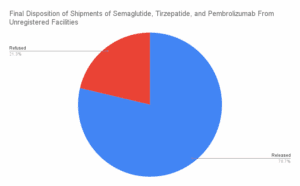
Read MoreWhat large-scale commercial imports made it into the country between March and August of 2025?
Read MoreThis summer, Brian Mayo, Executive Director of the Oregon State Pharmacy Association (OSPA), made the case for pharmacy benefit manager (PBM) reform as a better way to rein in drug prices.
Read MorePSM Executive Director Shabbir Safdar is speaking at the Democratic Governors Association about the threat of unregulated prescription drugs smuggled into the U.S.
Read MoreGilead Sciences filed a Lanham Act complaint against a group of companies that allegedly pushed patients into ordering non-FDA approved international medicines to lower costs in employer-sponsored health insurance plans.
Read MoreSupply chain complexity means that upper payment limits and Medicare maximum fair prices will fall disproportionately on pharmacies, leading to closures that threaten patient access to care.
Read MoreAlthough the state won FDA approval for its importation program in January 2024, it has yet to implement the program.
Read MoreMedical spas and wellness clinics are operating on the edge of existing regulatory frameworks and it’s a risk to American patients.
Read MoreBetween November 2022 and June 2023, Amarvel Biotech sold over 200 kilograms of precursor chemicals to U.S. buyers with full knowledge that they would be used to make fentanyl.
Read More