News
PSM endorses Iowa HSB 591 to strengthen med spa oversight for Iowans
The bill will protect Iowa’s patients by closing enormous regulatory gaps in the med spa and wellness industry.
[...]January 26, 2025: Health Canada warns Canadians about the dangers of unapproved GLP-1s
The agency has found websites and social media advertisements displaying official Health Canada logos with fake endorsements to mislead consumers, and said that it doesn’t endorse health products or permit its logo to be used in ads that promote them.
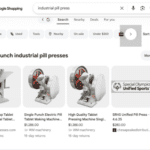
[...]January 20, 2026: PSM research leads Google to enforce its pill press ad policy
Reporting on PSM-led research led Google to suppress pill press ads, Congressman Raja Krishnamoorthi sent letters to shippers of weight loss drugs and API, and a former nurse was charged with distributing counterfeit Ozempic.
[...]In July, Google promised to rid their platform of criminals selling pill presses. How’s it going?
As of the first week of January, we found several companies with multiple ads still running well past its self-imposed deadline. Google can do better, but will they?
[...]FDA Alert: Supplements with undeclared drug ingredients
The FDA has posted notices for two nationwide dietary supplement recalls involving products found to contain undeclared pharmaceutical ingredients.
[...]January 12, 2026: New York Department of State warned consumers after citing 87 of 223 inspected med spas
New York found widespread safety violations in med spas, and FDA inspectors cited a Texas distributor for violating the DSCSA.
[...]Prescription Drug Freight Fraud Report, November 2025
Our analysis of data in September and October 2025 revealed that large-scale freight fraud involving GLP-1s, oncology drugs, and other high-demand pharmaceuticals continues unabated.
[...]January 5, 2026: CBP Cincinnati intercepted $407,000 in illegally imported medical products, including contact lenses, injections, and oncology drugs
CBP reported seizing 450 shipments of illegally imported contact lenses and prescription medicines. Dozens of state AGs asked Meta to enforce policies against misleading weight loss products.
[...]December 15, 2025: A Massachusetts man will plead to giving botulism-causing injections, says the U.S. Attorney’s Office
The med spa owner admitted to using unapproved injectables that sickened at least 10 patients. New York authorities announced the results of a 2024 crackdown on illegal med spas. PSM published in The Journal of Illicit Trade, Financial Crime, and Compliance.
[...]PSM urges the House Energy & Commerce Committee to support the SAFE Drugs Act of 2025
PSM wrote the House Energy & Commerce Committee in support of the Safeguarding Americans from Fraudulent and Experimental (SAFE) Drugs Act, to help protect the American drug supply from unsafely compounded medication.
[...]