About PSM
PSM Board Member Dr. Marv Shepherd Speaks with CBS News About Pharmacy Errors
When CBS Dallas reported on prescription fulfillment errors in Texas pharmacies, they reached out to The Partnership for Safe Medicine’s Board President, Dr. Marvin D. Shepherd to discuss the prevention of dispensing errors. Dr. Shepherd is Director of the Center for Pharmacoeconomic Studies and Chairman of the Pharmacy Administration Division at the University of Texas…
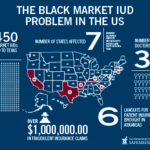
[...]PSM Releases Report on Black Market IUDs at U.S. Clinics
Between 2009 and 2014, 35 doctors in 7 states were prosecuted for exposing women to non-FDA approved IUDs. Doctors have been accused of purchasing IUDs from “Canadian” fake online pharmacies and importing untested IUDs from Mexico, then billing Medicare for the cost of genuine, FDA-approved IUDs. Over the same time period, authorities estimated that over 450 women were implanted with misbranded, black-market IUDs.
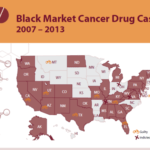
[...]PSM Releases Overview of Unapproved Cancer Medication Cases
PSM’s Black Market Cancer Drug Cases 2007-2013 looks back through the past 6 years at the numerous cases of fake or misbranded cancer drugs that were sold by shady black market drug sellers and purchased by doctors and clinics throughout the United States. Since 2007, 16 physicians and drug distributors have been prosecuted for their…
[...]Interchange 2013 Highlights: US Attorney Samuel Louis Describes How a Desire For Diamonds Brought a Master Counterfeiter to Justice
U.S.Attorney Samuel Louis speaking at Interchange 2013 We were fortunate enough to have US Attorney Samuel Louis share details from the counterfeit medication cases he has prosecuted at the Interchange 2013. During the course of his presentation, he described how master drug counterfeiter Kevin Xu was finally brought to justice. Kevin Xu was a businessman…
[...]Senate Passes HR 3240, “Drug Quality and Security Act”
PSM applauds the members of the Senate for passing HR 3204, "Drug Quality and Security Act." The securing of the supply chain through establishing track-and-trace requirements for medicine manufacturers, wholesalers and repackagers will provide Americans with greater security over our prescription drugs. When the supply chain is breached by someone mistakenly purchasing a substandard drug…
[...]Interchange 2013 Highlights: Dr. Marvin Shepherd of University of Texas at Austin Pharmacoeconomic Studies Program
Interchange 2013 Highlights: Dr. Patrick Lukulay of USP
USP has launched the Center for Pharmaceutical Advancement and Training in Accra, Ghana.Learn more about USP’s efforts to improve the quality of medicines globally at http://www.usp.org/around-world We were most fortunate to have Patrick Lukulay, PhD, Vice President of Global Health Impact Operations for the US Pharmacopeial Convention (USP) as a panelist at this year’s Interchange.…
[...]Partnership for Safe Medicine’s 4th Annual Medicine Safety Conference Success at Newseum in Washington, DC
On October 24th, the Partnership for Safe Medicines gathered stakeholders in the efforts to combat counterfeit medicine in Washington D.C. for a one-day conference at the Newseum’s Knight Conference Center. This was our 4th annual conference, focused on U. S. drug supply chain security and the safety of American patients. Speakers and panelists were drawn…
[...]Nemlekar P, Shepherd M*, Lawson K, and Rush S. Web-Based Survey to Assess the Perceptions of Managed Care Organization Representatives on Use of Copay Subsidy Coupons for Prescription Drugs. J Manag Care Pharm. 2013;19(8):602-08.
ABSTRACT BACKGROUND: Promotion of prescription drug coupons and vouchers by pharmaceutical manufacturers has increased in recent years. These coupons and vouchers usually subsidize patients’ cost-sharing obligations. In other words, drug companies pay for a patient’s portion of the drug cost, and the remaining cost is paid by the patient and the patient’s health plan. This…
[...]Will the PSM 2013 Interchange Still Happen During the Government Shutdown?
You can count on it. The criminals who counterfeit drugs and endanger American patients haven’t stopped making and selling fake drugs during the shutdown. There is more need for a counterfeit drug conference now than ever. The 2013 Interchange will include: Patient advocates who represent patients whose lives depend on getting genuine medication Journalists who…
[...]