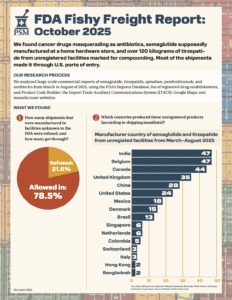
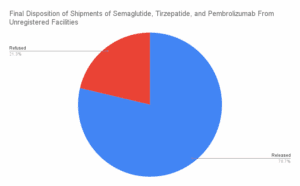
What large-scale commercial imports made it into the country between March and August of 2025?
This summer, Brian Mayo, Executive Director of the Oregon State Pharmacy Association (OSPA), made the case for pharmacy benefit manager (PBM) reform as a better way to rein in drug prices.
PSM Executive Director Shabbir Safdar is speaking at the Democratic Governors Association about the threat of unregulated prescription drugs smuggled into the U.S.
Supply chain complexity means that upper payment limits and Medicare maximum fair prices will fall disproportionately on pharmacies, leading to closures that threaten patient access to care.
Although the state won FDA approval for its importation program in January 2024, it has yet to implement the program.
Medical spas and wellness clinics are operating on the edge of existing regulatory frameworks and it’s a risk to American patients.
Between November 2022 and June 2023, Amarvel Biotech sold over 200 kilograms of precursor chemicals to U.S. buyers with full knowledge that they would be used to make fentanyl.
Our podcast covers the latest in pharma crime and medicine safety.
Like your information on video? Subscribe to our YouTube playlist!

Regulatory confusion leaves med spa patients at risk. Learn more.

Gaps in FDA oversight of 503B compounding facilities put patients at risk.

More fake Ozempic in the U.S. Learn more.
Click the images below to see more recent videos.