Hot issues
The operation launched 1,728 investigations in 91 countries and shut down 13,000 criminal-linked websites, social media pages, channels, and bots used to market and sell illegal or counterfeit medicines.
On June 26, PSM commented in support proposed revisions to forms FDA 3500 and FDA 3500B, which are used to report adverse events from prescription and over-the-counter medicines.
More than 40 cases of botulism linked to Botox injections have been reported in the U.S. and U.K. over the last two months.
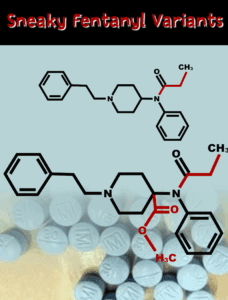
The bill permanently schedules fentanyl analogues as Schedule I drugs under the Controlled Substances Act.
The Jha brothers admitted selling counterfeit Keytruda and other prescription drugs to undercover agents.
PSM is soliciting feedback on draft best practices until June 20th. Please review the guidelines and send your thoughts to editors@safemedicines.org.
The FDA warned companies over the sale of an unapproved medical device and contaminated eye medicines, and disbarred a Texan who sold illegally imported pharmaceuticals.
Our podcast covers the latest in pharma crime and medicine safety.
Like your information on video? Subscribe to our YouTube playlist!

Who's investigating a company selling research-grade weight loss injections? Find out..

Updates on two federal prosecutions.

Click the images below to see more recent videos.