Unregulated pill presses and molds make fake pills with dangerous and even deadly consequences for thousands of people every year. Read our July 2025 to January 2026 update about pill press seizures, policy developments, and legislation.
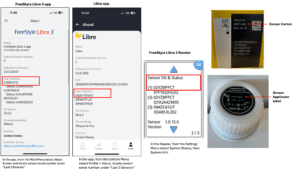
The FDA has posted a notice about the recall of continuous glucose monitors from Abbott.
The federal spending package that ended January’s government shutdown will prohibit PBMs from linking rebates to drug companies’ list prices. The FTC reported that PBMs inflated the prices of generic drugs.
The FDA has posted a notice for a dietary supplement recalls involving products found to contain undeclared pharmaceutical ingredients.
PSM Executive Director Shabbir Imber Safdar released the following statement in response to news that Hims & Hers is now compounding pill versions of a newly approved GLP-1 weight-loss drug.
This post outlines practical steps Congress should take to strengthen pharmaceutical border security, reinforce regulatory oversight, and protect patients.
A patient at a North Dakota med spa told investigators she had to coach the clinic owner through the IV administration process. The owner allegedly presented herself as a registered nurse, despite holding no medical licenses.
The bill will protect Iowa’s patients by closing enormous regulatory gaps in the med spa and wellness industry.
The agency has found websites and social media advertisements displaying official Health Canada logos with fake endorsements to mislead consumers, and said that it doesn’t endorse health products or permit its logo to be used in ads that promote them.
Reporting on PSM-led research led Google to suppress pill press ads, Congressman Raja Krishnamoorthi sent letters to shippers of weight loss drugs and API, and a former nurse was charged with distributing counterfeit Ozempic.