News Coverage
The Partnership for Safe Medicines has been publishing information about the counterfeit drug problem around the world for more than a decade. With experts leading the organization and a committed and passionate set of writers and editors, our content is more in-depth than many other sources, which simply copy links to the news from other websites.
A few weeks ago, Customs and Border Protection in Tennessee announced that it had intercepted counterfeit vaccine cards shipped from China. This week’s roundup adds fake card news in Alaska, Illinois and Florida. Catch up on these and more than a dozen other safe medicines stories.
Washington, D.C. (August 12, 2021) – Shabbir Safdar, executive director of the Partnership for Safe Medicines, released the following statement in response to President Biden’s remarks about lowering healthcare costs for Americans
An IT security firm reports that the number of sellers advertising fake vaccination cards on Telegram has spiked 257% since March 2021.
Catch up on this news and the rest of the week’s COVID-scam and counterfeit medicine news here.
Am eBay seller is voluntarily recalling all lots of Hydro Pineapple Burn to consumer level because FDA analysis has found the product to contain undeclared sibutramine, an appetite suppressant that was withdrawn from the market because of safety issues.
Je Dois L’avoir Boutique is voluntarily recalling all of the 365 Skinny High Intensity Pills and or 365 Skinny Emergency Boutique, 30 day capsules supply to the retail/consumer level. The 365 Skinny High Intensity Pills and 365 Skinny Emergency Boutique have been found to contain Sibutramine which is a controlled substance by the DEA.
There are a lot of ineffective and ridiculous #covidscams out in the world, but this particular scam the FDA found that offers to “cure” COVID-19 with magnets takes the cake. Watch “Save Me Magnets!” to learn about this and other harebrained scams. If you need protection from COVID-19, it’s FREE! Text your zip code to 438829 to find out the nearest place for you to get a free vaccine.
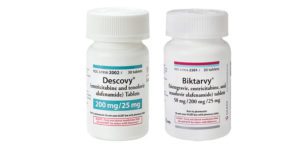
Gilead Sciences reports that unauthorized distributors have been selling counterfeit versions of its HIV treatments, Biktarvy and Descovy, to U.S. pharmacies. It’s the second case of counterfeit HIV medicine reaching U.S. pharmacies in the last 12 months.
Gilead has alerted potentially impacted pharmacies to investigate the potential for counterfeit or tampered Gilead medication sold by distributors not authorized by Gilead that may be within their recent supply and to remain vigilant to the potential for this to occur in the future. The authenticity and safety of Gilead-branded medicines can only be secure when obtained directly through Gilead’s authorized distributors.
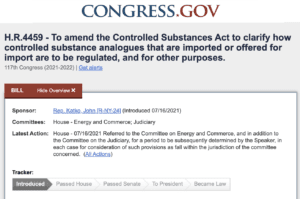
HR 4459, the Stop the Importation and Manufacturing of Synthetic Analogues (SIMSA) Act of 2021 would amend the Controlled Substances Act to “result in faster control of drugs designed to be used in the same illicit manner as already regulated or outlawed drugs.” Catch up on this and more than 30 more counterfeit medicine news stories.
Homeland Security Investigations’ Operation Stolen Promise has declared that its second year will focus on on fake treatments and vaccine fraud. That’s good, because this kind of fraud is pervasive, and it’s even coming from trusted sources like doctors. Learn about two recent federal cases here.
The U.S. Attorney’s Office in Maryland seized two websites that tried to mimic legitimate Walmart websites, and claimed to sell drugs that treat or prevent COVID-19. Read this and 15 more stories in the world of fake medicine.
In May, New Orleans-based WWL TV covered the death of a high school senior who was killed by a fake prescription pill made with fentanyl just days before her graduation. Getting news out about these pills is incredibly important but so is accuracy. PSM and a group of parents wrote the station to point out the danger of describing these deaths as overdoses rather than poisoning.
Shabbir Safdar, executive director of the Partnership for Safe Medicines (PSM), released this statement in response to the Centers for Disease Control and Prevention’s (CDC) preliminary statistics for the number of Americans whose deaths in 2020 involved drugs
Tragic preliminary statistics that show that drug deaths rose from 70,600 in 2019 to 93,000 in 2020 with 57,000 the result of synthetic opioids like fentanyl.
Click to read this and the rest of this week’s counterfeit medicine news.
Shabbir Safdar, executive director of the Partnership for Safe Medicines, released this statement in response to President Biden’s executive order to foster competition, which favors importation of prescription drugs from Canada.
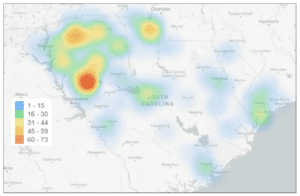
Learn about South Carolina’s grave warning for its residents, and more than 30 other stories in counterfeit news.
With the year half over, where are we with policy proposals and crimes that affect the safety of the supply chain?
Watch our June 30th webinar for an update.
CBP officers captured more than 100 shipments of black market versions of the Covid-19 treatment remdesivir in recent months. Read to learn more about this and the rest of the week’s news in fake medicine.
A guilty plea for a Connecticut man who made deadly counterfeit Percocet in Stamford, and a dozen more fake medicine stories.
On June 4, 2021, parents and family members gathered in 30 cities around the country to protest social media companies’ inaction on drug sales on their platforms. The protest in Santa Monica focused on Snapchat headquarters.
Every year, Interpol coordinates Operation Pangea, a seven-day initiative that targets the sale of counterfeit and unauthorized medical products. Read this to learn about this year’s fake medicine haul, and catch up on 17 other stories in the news.
Families rallied in 30 U.S. cities to draw attention to fentanyl poisoning deaths and to demand that social media companies stop drug dealers selling on their sites. Plus, 23 more counterfeit medicine stories.
What’s happening with Canadian drug importation? What’s Florida doing with a big empty warehouse? Isn’t this policy currently in the courts? You’ve got questions and we’ve got answers.
Customs and Border Protection are hard at work protecting Americans from counterfeit medicines and fake medical devices. Read on to learn about recent seizures of prescription steroids, COVID-19 test kits and more in Cincinnati, and catch up on 27 other stories about medicine safety.
A Mississippi physician will pay more than half a million in fines, forfeitures and restitution for treating his patients with foreign, non-FDA-approved versions Prolia, Boniva, and Aclasta.
And 15 more stories from the world of counterfeit medicine.
The media speaks as if counterfeit pill victims were seeking drugs and “overdosed” because they took too much of them, but that doesn’t accurately reflect what is happening. Too often, victims have been sold drugs that they weren’t seeking at all.
Help PSM tell journalists that pill victims have been poisoned, not overdosed.
U.S. Attorneys in California announced 12 new drug prosecutions (8 for fake fentanyl pills) all of which involved deaths. Plus, 19 more stories about counterfeit medicines and COVID-19 fraud.
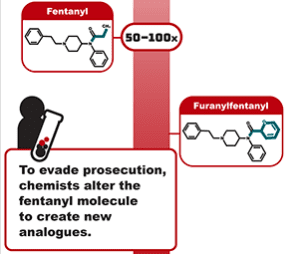
A short 6-month extension for emergency scheduling of fentanyl analogues, an update to Illegal Pill Presses: An Overlooked Threat to American Patients, and twenty more COVID fraud and medicine safety stories.
The Partnership for Safe Medicines and the National Association of Drug Diversion Investigators have released an update to 2019’s Illegal Pill Presses: An Overlooked Threat to American Patients.
Since the initial report, fentanyl deaths are higher than ever and these pills – created by clandestine pill presses around the globe – continue to be sold on the streets and on the dark web.
This week’s roundup includes: Fake remdesivir plagues India and 18 more counterfeit medicine and COVID-fraud stories you may have missed.
Shabbir Safdar, Executive Director of the Partnership for Safe Medicines, released the following statement today in response to Congressional actions to permanently scheduling illicitly manufactured and deadly fentanyl…
This week’s roundup includes: a conviction in Colorado, fake COVID-19 vaccines, Operation Stolen Promise statistics and 17 more counterfeit medicine stories.
This week: 70 months for a Pennsylvania man who trafficked counterfeit medicines, two fentanyl pill operations shut down, counterfeit pill seizures and warnings and a couple of #covidscams.
ADAP Advocacy Association CEO Brandon M. Macsata wrote this editorial, which appeared on the AIDS Drug Assistance Program’s website on April 1, 2021.
The Association first warned constituents that counterfeit Symtuza had been distributed to three U.S. pharmacies in December 2020, when Janssen issued an alert.
This week: The government undertakes scheduling fentanyl analogues, another silicone injection case, a huge counterfeit pill trafficking bust in Quebec, and many, many more fentanyl pills.
Learn why permanently scheduling fentanyl-related substances and analogues on Schedule I of the Controlled Substances Act is an important policy tool in the fight against fentanyl-laced counterfeit medicines.
PSM wanted to shine a spotlight on member organization Rx Outreach. Not only does this organization provide affordable medications for people in need, but Rx Outreach also runs a program called Healthy Reentry to fill the medication gap to help ex-offenders as they transition back into society…
This week: A federal jury indicts a man for smuggling and selling fraudulent COVID-19 treatments, a woman pleads guilty for a silicone injection death in Missouri, police seize nine million fake clonazepam tablets in Europe, and news about counterfeit pills in ten U.S. states.
This week: A new contaminant in hand sanitizers, mystery medicated eye patches, sentencing for Ukrainian counterfeiters and counterfeit pill updates.
On December 17, 2020, FDA issued a press release that warned consumers to avoid certain products found on Amazon, eBay and other retailers due to hidden and potentially dangerous drug ingredients. It also encouraged online marketplaces to ensure these products are not sold on their platforms.
On December 17, 2020, FDA issued a press release that warned consumers to avoid certain products found on Amazon, eBay and other retailers due to hidden and potentially dangerous drug ingredients. It also encouraged online marketplaces to ensure these products are not sold on their platforms.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using these drug products.
This week: fake COVID-19 vaccines seized in Mexico, updates on the impact of fentanyl pills in 2020, warnings about counterfeit pills made with methamphetamine and more.
The White House approved regulations for state drug importation in late state, green-lighting several states’ efforts to implement importation.
None of them is up and running yet. Check out this week’s video and post for an update.
This week: FDA warns against ivermectin as a COVID treatment, a fourth fraudulent pharmaceutical company website, counterfeit pill prosecutions and seizures in seven states.
This week: The FDA moves on fake FDA registration certificates and warns Mercola to stop selling supplements with fake COVID claims. The U.S. Attorney in Maryland shuts down another fake pharma phishing site. Counterfeit vaccines in South Africa. Counterfeit pill news in 11 states.
The annual DEA National Threat Assessment, which covers 2019 and the first half of 2020, is the best source of unclassified data on drug smuggling, illicit substance trafficking, and counterfeit medications as it affects Americans. Watch our News of the Week video at right, or read the high points on our Twitter thread. Below you’ll…
The CDC says drug deaths are up 24% and the U.S. has seized nearly one million fake pills in the last 30 days.
This week: More counterfeit medical masks in Washington; prosecutions in nine states; fentanyl pill poisoning deaths in California, Missouri, New York, North Dakota and Wisconsin; and more.
In this week’s news: 11.1 million counterfeit n95 masks seized, invalid COVID-19 test results, pandemic-related benefits fraud, large fentanyl pill seizures, and another two doctors plead guilty to importing oncology drugs.
In this week’s news: More counterfeit masks, more fake medicine seizures, and a deadly new ingredient in pressed pills: clonazolam.
This recall has been initiated after an FDA laboratory analysis found the product to contain undeclared sildenafil and/or tadalafil. Sildenafil and tadalafil are ingredients in FDA approved products for the treatment of male erectile dysfunction in the family of drugs known as phosphodiesterase (PDE-5) inhibitors. The presence of sildenafil and/or tadalafil in Adam’s Secret Extra Strength products makes them unapproved new drugs for which the safety and efficacy have not been established and therefore subject to recall.
In this week’s news: Counterfeiting of COVID-19 masks, vaccines and medicines continues; significant fake medicine operations in Canada, Mexico, the European Union, and here in the U.S.
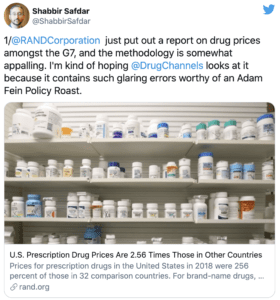
In 2019, the U.S. Health and Human Services Agency hired the RAND Corporation to do a study comparing drug prices around the world to U.S. prices. They found that Americans pay 16% less than other countries for our unbranded generics—and generics make up 85% of drugs Americans are prescribed.
In this week’s news: Fake masks, fake COVID vaccines, fake pills and fake pill manufacturing across the U.S.
Under the import alert, alcohol-based hand sanitizers from Mexico offered for import are subject to heightened FDA scrutiny, and FDA staff may detain the shipment. As part of their entry review, FDA staff will consider any specific evidence offered by importers or manufacturers that the hand sanitizers were manufactured according to U.S. current good manufacturing practice requirements. This marks the first time the FDA has issued a countrywide import alert for any category of drug product.
When the first FDA alerts warning of methanol-contaminated hand sanitizers brands emerged, we reported them through our FDA alert system. Within weeks however, this trickle of hand sanitizer recalls turned into a flood. Learn more here.
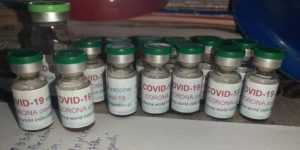
In this week’s news: A Seattle man was arrested for COVID-vaccine fraud; sales of fake COVID-19 vaccines on the dark web up 400% since December; prosecutions of fake pill peddlers, and more.
This week: A new report from the National Association of Boards of Pharmacy, COVID-19 vaccine fraud, a guilty plea in a fake online pharmacy case, counterfeit pill news, and more.
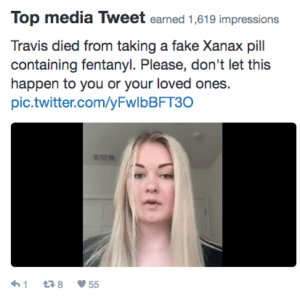
When the Central Valley Opioid Safety Commission contacted us about helping them promote a public safety message about the dangers of counterfeit pills made with fentanyl, of course we said yes. Their 30-second spot featured the family of Travis Jacobson, a young Californian who was tragically killed by a fake Xanax pill that turned out to be lethal. We at PSM were happy to underwrite both the direct costs and the labor costs of such an important public health message.
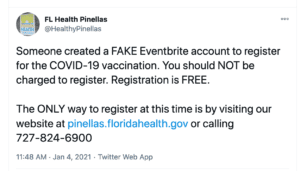
PSM’s round-up this week closes out 2020 with hundreds of thousands of counterfeit surgical masks, scammers cashing in on COVID-19 vaccines with fake Eventbrite listings, fake cancer treatments, and more.
Watch our two minute video and read this post to learn how Florida’s importation plan fails to meet federal requirements, its own requirements, and promises supporters made during the legislative process.
24-year-old Taylor Martinek died of fentanyl poisoning in 2017, after he took an oxycodone pill that turned out to be counterfeit. Since then, his family has been trying to change Oregon law to increase penalties for dealers who cause deaths like Taylor’s.
This is a reprint of an FDA Alert. Essaar Inc. Issues Voluntary Nationwide Recall of Rubbing Alcohol Contaminated with Methanol When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does not endorse either the product or the company. Company Name:Essaar Inc.Company Contact…
If you’re a reporter who covers healthcare and especially public health issues, this is what we think you should be watching for in the next six months of a better 2021
In PSM’s round-up this week: Make sure you’re getting the REAL COVID-19 vaccine, plus prosecutions and seizures involving dangerous supplements, a fake online pharmacy and a variety of counterfeit pills.
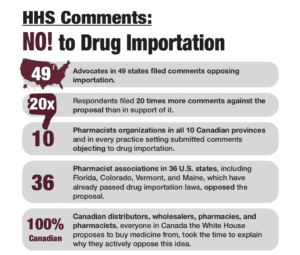
Many advocates and legislators are considering whether to pursue Canadian drug importation in 2021, but importation is not and never has been a viable option.
Watch our video to learn the basics and for an update on four states that have been trying to move forward with this idea.
In PSM’s round-up this week: ongoing COVID-19-related fraud, Europol’s Operation Shield, and counterfeit pill news in six states.
The Partnership for Safe Medicines (PSM) applauds the passage of H.R. 5663 – the Safeguarding Therapeutics Act. The bipartisan bill, introduced by Congressman Brett Guthrie (KY-02-R) and Congressman Eliot Engel (NY-16-D), was passed via unanimous consent in the Senate on December 8th.
This editorial by the Sun Sentinel Editorial Board was published in The Sun Sentinel on December 9, 2020. The Editorial Board consists of Editorial Page Editor Rosemary O’Hara, Dan Sweeney, Steve Bousquet and Editor-in-Chief Julie Anderson. There Go Those Cheaper Drugs. Remember Florida’s plan to save money by importing prescription drugs in bulk from…
In PSM’s round-up this week: Canada banned exporting drugs to the U.S. if it will cause drug shortages at home, the FTC warns about fake COVID-19 testing centers, several large counterfeit pill busts, and more.
On Tuesday, December 2, 2020, New Mexico conducted their one and only public hearing on their plan to import medicine from Canada (over Canada’s objections). While the hearing was largely a formality, there was some interesting testimony.
Would you fly 6,000 miles to meet with a stranger who could be your new literal partner in crime? In April 2019, two Ukrainians did just that. While they dreamed of getting their counterfeit medicines into the legitimate U.S. drug supply chain, that dream was thwarted by the hard work and perseverance of federal investigators in Immigration and Customs Enforcement’s Homeland Security Investigations (HSI).
In an analysis of 50 prospective drugs, Maine’s Medicaid program found that it would lose nearly a million additional dollars ($927,983.28, to be exact) importing these drugs from Canada. How can this be? For many reasons, Canada is not the land of cheap drugs people think it is.
Today, PhRMA, The Partnership for Safe Medicines and Council for Affordable Health Coverage initiated litigation in the U.S. District Court for the District of Columbia challenging action by HHS and FDA permitting pharmacists and wholesalers, pursuant to state-sponsored programs, to import certain prescription drugs from Canada into the United States without drug manufacturers’ authorization or oversight.
In PSM’s round-up this week: Government authorities take legal action against COVID scammers; an insurance broker (allegedly) decided to open his own unlicensed pharmacy; ongoing counterfeit medicine news, and more.
In PSM’s round-up this week: An analysis of New Mexico’s draft drug importation plan, reports on COVID-19 fraud, and this week in counterfeit medicine news.
The state of New Mexico released a draft of its Canadian drug importation plan. PSM analyzed the plan, paying particular attention to concerns pharmacists might have about how drug importation might affect their patients and business…
In PSM’s round-up this week: FDA and FTC warnings over fake COVID-19 treatments and fraud, as well as fake medicine news in seven U.S. states, Canada and Cambodia.
Seeking a good night’s sleep, 25-year-old Jake Beddoe, a young travel consultant with an adventurous heart and a tremendous sense of humor, took part of what he thought was a Xanax pill on May 27, 2020. The pill was counterfeit, and Jake died of fentanyl poisoning.
In PSM’s round-up this week: news about state drug importation, COVID-19 fraud, fake hand sanitizer stations, and more counterfeit medicine news.
This editorial by Brandon Macsata was published in The International Business Times on November 1, 2020. Macsata has been living with HIV since 2002, and serves as the CEO of the ADAP Advocacy Association, an organization that promotes the AIDS Drug Assistance Program and works to improve access to care.
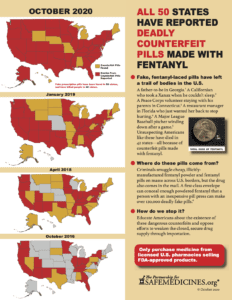
With the recent report that police in Maui, Hawaii seized 400 counterfeit oxycodone pills made with fentanyl on October 2, 2020, the United States has reached a sobering milestone: public sources have reported about fake pills made with fentanyl in all 50 states.
In PSM’s round-up this week: news about state drug importation, COVID-19 fraud, deadly workout supplements and counterfeit pills made with fentanyl.
A summary briefing of 2020’s major cases, worrying trends and a deep dive into drug supply chain policy issues.
In PSM’s round-up this week: new reports about counterfeit medicine, drug supply chain regulation and phishing scams; FDA warnings about dangerous supplements; a life sentence for fake pill kingpin Aaron Shamo; and more.
In this analysis, which was published in Lexology on October 13, 2020, three global regulatory experts examine barriers to drug importation.
In PSM’s round-up this week: Policy truths about drug importation, prosecutions for fake COVID-19 treatments and financial fraud, and another week in counterfeit pills.
In the first seven months of 2020, the Arizona Department of Health Services reported over 2,700 newly suspected opioid overdose deaths in Arizona. What makes this figure more startling is that there were just over 3,800 suspected opioid deaths in the preceding 30 months. The problem is escalating, and rapidly.
The editorial board of the Florida Times-Union published an op-ed that states that Canadian drug importation will have a limited impact, and is a sign of a broken system, not a solution to one…
Wyoming is one of many states that decided to examine if importing drugs would provide significant savings to its citizens. In a hearing in August and a report released today, they took a critical look at the issue and the Department of Health recommends that the state not pursue the idea for several key reasons…
In PSM’s round-up this week: A new report about the counterfeit medicine trade, fake COVID-19 vaccines in India, the week in counterfeit pills.
In PSM’s round-up this week: HHS defies 20 years of expertise and guidance and recent negative feedback to finalize regulations for state-based Canadian drug importation programs, forward movement on the Safe Therapeutics Act, and more #covidscam and counterfeit drug news.
On September 23, 2020, Department of Health and Human Services Alex Azar submitted a letter to House Minority Leader Kevin McCarthy certifying that Canadian drug importation would pose not additional threats to consumers and would save Americans a significant amount of money on prescription drugs.
Trump Administration Defies 20 Years of FDA Expertise and Guidance in Finalizing Politically Motivated Drug Importation Rule Washington, D.C. (September 24, 2020) – Shabbir Safdar, executive director of the Partnership for Safe Medicines, released the following statement in response to the Trump Administration’s Final Rule issued today on the importation of prescription drugs: “With 40…
On September 24th, 2020 the U.S. Department of Health and Human Services released a draft of finalized rulemaking for Importation of Prescription Drugs. Watch this space for the most up-to-date news.
Last June, police in Ontario, Canada carried out Project Javelin, shutting down a huge counterfeiting operation that manufactured hundreds of thousands of fentanyl pills disguised as Oxycocet, a Health Canada-approved generic oxycodone and acetaminophen product with the same ingredients as Percocet.
Update: Executive Director Shabbir Safdar followed up with retired Chief Superintendent of the Ontario Police Don Bell to talk about the impact these criminals could have had. Watch that interview in our September 23rd video, “Deadly Pills in Ontario.”
In PSM’s round-up this week: 520,000 fake medical masks seized by Customs and Border Protection, another fake online pharmacy case, and a flood of news about counterfeit pills made with fentanyl.
Risk Statement: Patients being treated for hypothyroidism (underactive thyroid), who receive sub potent NP Thyroid®, may experience signs and symptoms of hypothyroidism (underactive thyroid) which may include, fatigue, increased sensitivity to cold, constipation, dry skin, puffy face, hair loss, slow heart rate, depression, swelling of the thyroid gland and/or unexplained weight gain or difficulty losing weight. There is reasonable risk of serious injury in newborn infants or pregnant women with hypothyroidism including early miscarriage, fetal hyperthyroidism, and/or impairments to fetal neural and skeletal development. In elderly patients and patients with underlying cardiac disease toxic cardiac manifestations of hyperthyroidism may occur, such as cardiac pain, palpitations or cardiac arrhythmia. To date, Acella has received four reports of adverse events for these lot numbers possibly related to this recall.
In PSM’s round-up this week: $132 million in losses from #covidscams since March, fake online pharmacies selling counterfeit and non-FDA approved opioids, Operation Quack Hack, and more.