HHS's Federal Action Plan for Drug Importation

On May 24, 2022, PSM held an online congressional briefing with a panel of experts to let legislators know why drug importation is a dangerous proposition. Watch briefing highlights or the entire event.
Current status:
The regulations took effect on November 30, 2020. PSM, PhRMA, and CAHC have filed suit in federal court to block them from taking effect.
On May 28, 2021 the Department of Health and Human Services filed a motion to dismiss the suit on the grounds that PSM, PhRMA, and CAHC did not have grounds to sue until the government had approved a state importation program.
PSM, PhRMA, and CAHC filed an amended complaint on July 2, 2021.
In the meantime, Colorado and Florida have submitted applications to begin state importation programs.
The State of Florida and Florida's Agency for Health Care Administration filed suit against the FDA and Department of Health and Human Services for delaying approval of its state importation program in August 2022.
Synopsis:
On September 24, 2020, the federal government released a Final Rule on Canadian drug importation (pathway 1), as well as Industry Guidance for manufacturer reimportation (pathway 2), and requests for proposals for waivers for individual prescription drug importation (pathway 3) and insulin reimportation programs (pathway 4) with an accompanying FAQ for the latter.
PSM, PhRMA, and CAHC have all filed suit against these regulations. You can read our joint announcement, our explainer about why we filed the litigation, and the complaint itself [PDF].
How should we evaluate this program?
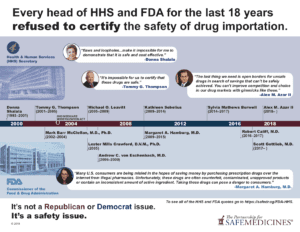
Until July 2019, every head of Health and Human Services and the FDA since 2003 has refused to certify the safety of drug importation. Many—including Alex Azar, former FDA Commissioner and Trump appointee Scott Gottlieb, and his four immediate predecessors—have explicitly criticized these proposals as unsafe, unimplementable, and unlikely to save money.
HHS and FDA haven't implemented their proposals yet so there's no way to evaluate whether they save money or keep patients safe. However previous programs in Maine, Illinois, and Minnesota all shut down because they didn't save the money promised. They all had safety lapses as well.
Official Actions Since the Rule was Approved
June 1, 2021: Florida files an amicus brief in support of HHS's importation rule.
May 28, 2021: HHS files a motion to dismiss the suit.
November 23, 2020: PSM, Pharmaceutical Research and Manufacturers of American and The Council for Affordable Healthcare file a complaint alleging that the Final Rule disregards key protections of the Federal Food, Drug, and Cosmetic Act
Timeline of the approval of final rule for Canadian drug importation
September 24, 2020 - HHS published final rule for Canadian drug importation, as well as RFPs for personal importation waivers and and reimportation of insulin, and guidance for manufacturers who may choose to reimport their own drugs. (Links to those documents below, in Planning Documents.)
December 23, 2019 - March 9, 2020: HHS solicits comments about RIN 0919-AI45
July 31, 2019:
- HHS Announces New Action Plan to Lay Foundation for Safe Importation of Certain Prescription Drugs
- Remarks on the Safe Importation of Certain Prescription Drugs
August 6, 2019:
August 20, 2019:
- Florida submits a Canadian Prescription Drug Importation Concept Paper to HHS
Fall 2019:
- Office of Information and Regulatory Affairs posts RIN 0910-AI45, a draft of the proposed rule for the importation of prescription drugs.
Planning documents
Pathway 1, Wholesale Canadian Drug Importation: Final rule (PDF, 179 pages) and Redline (shows differences from December's draft rulemaking).
Pathway 2, Manufacturer Reimportation: Guidance for Industry
Pathway 3, Individual Waivers for Personal Importation: Request for Proposals
Pathway 4, Reimportation of Insulin: Request for Proposals and FAQ
Letter from HHS Secretary Alex Azar to House Minority Leader Kevin McCarthy certifying the final rule on wholesale Canadian drug importation, September 23, 2020.
HHS/FDA announces the Safe Importation Action Plan. July 31, 2019
Section 804 of the Food, Drug and Cosmetic Act which outlines the method by which the Federal government may import medicine from Canada.
Background / resources
Just learning about foreign drug importation proposals? Start with some of these resources that outline the safety issues.
PSM Materials:
- Learn about failures to save money and keep patients safe in previous importations programs in Maine, Illinois, and Minnesota.
- Every head of Health and Human Services and the FDA since 2003 has refused to certify the safety of drug importation
- Importation has been opposed by dozens of groups representing law enforcement, patients, regulators, and healthcare professionals for nearly two decades.
- Drug importation endangers U.S. patients by breaking our closed, secure drug supply
- Common misconceptions about drug importation
Challenges to importation:
- Canadian patient groups, healthcare groups, boards of pharmacy and the Canadian government have not agreed to importation.
- A 2018 study by PSM board member and pharmaco-economist Dr. Marv Shepherd shows that if 20% of U.S. prescriptions were filled using Canadian prescription drug sources, the Canadian drug supply would be exhausted in 183 days.
- Dr. Kristina Acri's analysis shows that importation programs are unlikely to save money because of the costs of testing medication and treating patients who encounter counterfeits.
- Importation breaks Track and Trace systems which have been in-process since 2013, when Congress passed the Drug Supply Chain Security Act.
- State Drug Importation Laws Undermine the Process That Keeps Our Supply Chain Safe (July 11, 2019)
- In February 2017, Alan Coukell, the senior director of health programs for the Pew Charitable Trusts wrote Senator Bernie Sanders, to raise concerns about the effect of importation on the pharmaceutical supply chain security provisions.
- To learn more about Track and Trace, consult the FDA and RXTrace
Coverage:
- Everything (or almost everything) you need to know about importing drugs from Canada, July 31, 2019
- Canada wasn’t consulted on specifics of U.S. plan to import prescription drugs, July 31, 2019
- U.S. demand is threatening Canada's drug supply, groups warn feds, July 26, 2019
- Exclusive: Canada warns U.S. against drug import plans, citing shortage concerns, July 18, 2019
- HHS Secretary Alex Azar dismisses drug importation as a gimmick, May 14, 2018
Op-eds from the Experts
Former DEA agent Doug Herber wrote this editorial, which was published on May 31, 2019 in the White Mountain Independent. In it, he writes that drug importation will cause “patients [to] unwittingly purchase foreign counterfeit drugs disguised as low-level medication, unaware of the dangers, end up as an overdose statistic. “
This editorial by Holly Strom and Kenneth Schell published in U.S. News and World Report on May 28, 2019 warns states considering drug importation that doing so will not keep costs down and also poses a safety risk to patients…
In this editorial, which was published in The Bend Bulletin on May 21, 2019, Canadian law enforcement veteran Don Bell explains why Oregonians can not rely on Canada for safe prescription drugs.
Dozens of the most highly qualified law enforcement officials and former, senior staff at the U.S. Food & Drug Administration have conducted in-depth analyses that show Canadian drug importation will lead to a massive increase in counterfeit drugs entering the U.S.
In this editorial published in The Hill on May 12, 2019, Brooklyn Roberts, the director of the health and human services task force at the American Legislative Exchange Council, discusses the risks of drug importation:
“The safety of our prescription drugs relies on a closed system where drugs can be traced to manufacturers, distributors, pharmacies and patients. Opening that system to foreign drugs would allow the potential for dangerous and potentially deadly medicines to land in the hands of the American public.”
In this editorial published on April 30, 2019, former FBI Director Louis Freeh talks about the safety risks of drug importation: “There are hidden risks and costs associated with the scheme that have not been getting much attention which impact your health and Colorado law enforcement’s ability to keep us safe.”
In this editorial published on April 26, 2019, Douglas Holtz-Eakin, economist and President of American Action Forum, questions economic truths about drug importation:
“Drug reimportation has long been the fool’s gold of health policy, and the Florida bill is no different. It flunks a basic policy analysis. But most amazing, it is drafted to raise hope, but not actually help Floridians.”
In this piece published in the Washington Free Beacon on April 25, 2019, staff writer Charles Fain Lehman explores issues around Florida’s drug importation proposal. “Critics,” he notes, “fear that the actual realities of regulatory oversight—especially in the hand of an as-yet-unnamed private vendor—will simply be too challenging to manage responsibly.”
In this piece, which was published in The Deseret News on April 24, 2019, pharmacist and Utah State Senate Majority Leader Evan Vickers raises serious concerns about importation as a strategy to lower drug prices:
“Anyone who truly understands how drugs are sold and distributed in the U.S. knows that there are very solid technical reasons that such importation is not viable. There are also serious concerns about drug safety, since the CHS cannot guarantee origin and purity on foreign-sourced drugs.”
In this piece, which was published on the ABC affiliate WJLA’s website on April 25, 2019, political analyst Boris Epshteyn explains that “this is a risky plan that will make it difficult to ensure that Floridians are receiving real and safe medicine.”