Search:
Filter:
Sort:
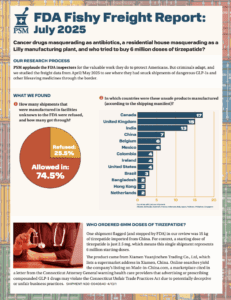
Our analysis of data from June and July revealed hundreds of pharmaceutical shipments that entered the U.S. from facilities no one would expect, including unregistered Chinese exporters and alternative medicine clinics abroad.
Read MoreThe warning letter was one of about 100 is a new focus on deceptive direct-to-consumer drug advertisements.
Read MoreThe “green list” will help protect patients from unreliable, illegally compounded GLP-1s and simplify screening for border security.
Read MorePSM led a coalition of organizations urging leaders of the Senate HELP and House Energy and Commerce committees to help strengthen the FDA’s ability to protect Americans from unsafe and counterfeit medicines and medical products.
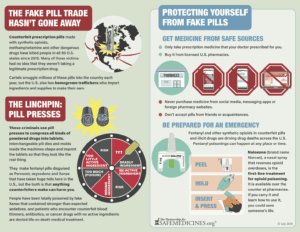
Read MoreA decade ago no one had heard of deadly counterfeit prescription pills. Victims’ families bravely spoke up, lobbied, and rallied for change.
Read MoreChinese companies that supplied U.S. compounders have turned to making generic semaglutide for markets where Novo Nordisk’s main patent is expiring in 2026.
Read MoreUnited States District Court District of Massachusetts USA v Rebecca Fadanelli Affidavits Filed October 2024 Read the documents: 1 | 2
Read MoreUnited States District CourtDistrict of Massachusetts USA v Rebecca Fadanelli Indictment Filed November 2024 Read the document.
Read MoreElle published an exposé about bad actors and loose regulation in the medical spa industry.
Read MoreUnited States District Court Western District of Washington USA v Avanish Kumar Jha Judgment Filed July 2025 Read the document.
Read More