Search:
Filter:
Sort:
Prescription Drug Affordability Board Activity, May 2025 Activities Summary Colorado: Colorado’s PDAB met on May 23 to approve the Data Submission Guide and begin rulemaking around an upper payment limit for Enbrel. Oregon: At its May 21 meeting Oregon’s PDAB discussed the timeline and process for affordability reviews and approved the generic drug report. One board member proposed looking beyond…
Read MoreThe FDA warned companies over the sale of an unapproved medical device and contaminated eye medicines, and disbarred a Texan who sold illegally imported pharmaceuticals.
Read MoreThe FDA has announced a voluntary nationwide recall of two dietary supplements sold exclusively online by www.umary-usa.com. Lab testing revealed that Unavy Ácido HIALURÓNICO and Umovy Ácido HIALURÓNICO contain undeclared active pharmaceutical ingredients: diclofenac, dexamethasone, and omeprazole.
Read MoreFederally-registered compounding facilities stopped making tirzepatide on May 22. This transition is significant for patients, and we at PSM think there are four things you should be watching for.
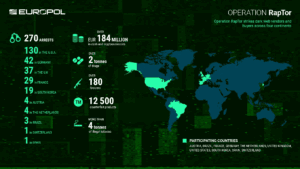
Read MoreOperation RapTor led to 270 arrests and the seizure of more than $200 million in currency and digital assets.
Read MoreArticles in Endpoints News and the Houston Chronicle raise questions about the regulatory process for 503B compounding facilities.
Read MoreExposes in the Houston Chronicle and Endpoints News examined Empower Pharmacy’s safety record. A grand jury indicted a Chinese company and three of its employees for pill press and counterfeit die mold sales.
Read MoreIn May 2025, the U.S. District Court in the Western District of Texas unsealed an indictment against a CapsulCN International and three Chinese nationals for their alleged role in the illegal importation of pill-making equipment.
Read MoreThe FDA wants to ensure that domestic and foreign companies receive the same level of regulatory oversight.
Catch up on research PSM released about medicines imported from unregistered sources.
Read More