News
November 24, 2025: Surveys examine online pharmacy use and patients’ perceived safety
Surveys document consumer behaviors when purchasing medication from online pharmacies, finding that the public may not be aware of the dangers of IOPs.
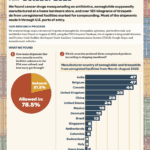
[...]Five takeaways from CNBC’s investigation of alternative funding programs
What are the most important lessons from CNBC’s documentary about alternative funding programs (AFPs)?
[...]November 17, 2025: CNBC explores “alternative funding programs” forcing U.S. patients to take unregulated medicines
CNBC aired a documentary about alternative funding programs that included interviews with PSM’s Shabbir Imber Safdar, HSI and FDA officials, as well as a representative from Gilead Sciences and from the companies supplying these drug.
[...]November 10, 2025: FDA cracks down on sites supplying unapproved “Botox”
The FDA warned websites in five countries to stop selling Americans unapproved cosmetic injections.
[...]PSM asks House Energy & Commerce Committee to oppose H.R. 5316, The Drug Shortage Compounding Patient Access Act
PSM sent a letter explaining how H.R. 5316 undermines critical patient safety protections.
[...]November 3, 2025: Safe Chain’s Boyd brothers convicted for selling adulterated HIV medicines and falsifying drug pedigrees
The Maryland distributors were convicted at trial in Florida. A federal judged sentenced a pill press seller from China.
[...]October 27, 2025: U.K. regulators bust illicit site making unlicensed retatrutide and tirzepatide pens
Authorities stated this was the largest single seizure of trafficked weight loss medicines worldwide.
[...]October 20, 2025: PSM leadership lends their expertise to scholarly journals
Board member Dr. Kenneth McCall was named an associate editor of the Journal of Pharmacy and Pharmaceutical Sciences; Executive Director Shabbir Safdar joined the editorial board of the Journal of Illicit Trade, Financial Crime, and Compliance.
[...]October 15, 2025: A JAMA study promotes med spa regulation; a new documentary explores fake GLP-1s
A study says IV hydration spas need more oversight. Louisiana residents were hospitalized after receiving black market botulinum toxin injections. Hulu delved into the counterfeit GLP-1 market and Fierce Healthcare covered AFPs.
[...]Prescription Drug Freight Fraud Report, October 2025
What large-scale commercial imports made it into the country between March and August of 2025?
[...]