Article Topic
Information, USA v Facial Expressions June 2025
United States District Court Eastern District of Kentucky Southern Division, London USA v Facial Expressions Information Filed June 9, 2025 Read the document.
[...]Prescription Drug Affordability Board Activity, July 2025
Prescription Drug Affordability Board Activity, July 2025 Activities Summary Colorado: Colorado’s PDAB met on July 11, 2025 for a rulemaking hearing to set an upper payment limit for Enbrel, during which they heard substantial public comment. They will continue this process at their next meeting on August 22. Maryland: At its July 28 meeting, Maryland’s…
[...]August 18, 2025: PSM, ADAP ask New York’s Board of Pharmacy to act in alleged fake medicine case
On August 7, the Partnership for Safe Medicines and ADAP Advocacy Association filed a complaint asking the New York State Board of Pharmacy to take regulatory action against a pharmacy that allegedly sold counterfeit HIV medicine to a Queens, New York patient.
[...]Tell us about your alternative funding program
Used an alternative funding program? Tell us your story The Partnership for Safe Medicines is researching alternative funding programs. If you are a patient or an administrator, we’d like to confidentially interview you to understand how you have encountered these programs, how they work, and what your experience was. If you’re ready to interview, or…
[...]August 4, 2025: Pennsylvania pharmacy owner indicted and 14 licenses revoked in Ohio
The Pennsylvania Attorney General filed charges against a pharmacy owner and the Ohio Board of Pharmacy revoked licenses to protect patients.
[...]July 28, 2025: Suspicious shipments of API continue to be sent to buyers in the U.S.
The newest Fishy Freight report continued to find suspect API shipped into the U.S. while a report from the United Nations’ Office of Drug Crime highlighted the dangers of contaminated and counterfeit medicines made with diethylene glycol and ethylene glycol.
[...]Prescription Drug Freight Fraud Report, July 2025
What if your tirzepatide shipment came from a Brazilian beauty clinic? Or a vial of semaglutide was manufactured, supposedly, at a Costco in Toronto? In April and May 2025, dozens of shipments of semaglutide, tirzepatide, apixaban, and antibiotics entered the U.S. from facilities that aren’t in the FDA’s drug manufacturing database. These aren’t low-volume personal-use…
[...]Final publication of guidelines for online pharmacy-to-pharmacy medicine marketplaces
We’ve published the final document of best practices for online pharmacy-to-pharmacy marketplaces.
[...]Partnership for Safe Medicines Applauds Reintroduction of the Cooper Davis Devin Norring Act
The Partnership for Safe Medicines (PSM) today announced its strong support for the reintroduction of the Cooper Davis Devin Norring Act, a bipartisan effort aimed at curbing the online sale of deadly counterfeit and illicit drugs.
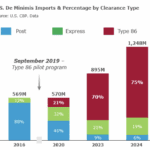
[...]July 21, 2025: Inspection policy for all FDA-regulated products changed at ports of entry
A policy change announced by U.S. Customs and Border Protection means that all FDA-regulated products are now subject to inspection at ports of entry.
[...]