Elle published an exposé about bad actors and loose regulation in the medical spa industry.
On August 7, the Partnership for Safe Medicines and ADAP Advocacy Association filed a complaint asking the New York State Board of Pharmacy to take regulatory action against a pharmacy that allegedly sold counterfeit HIV medicine to a Queens, New York patient.
PSM and ADAP Advocacy Association filed an official complaint with the State of New York regarding allegations that City Plus Care Pharmacy Inc. dispensed counterfeit HIV medication to a patient in Queens, New York.
Statistics in the agency’s Report on the State of Pharmaceutical Quality show that active pharmaceutical ingredient (API) sites that solely supply compounding pharmacies are disproportionately high risk.
The proposed updates aim to help address safety concerns created by pharmacies reselling compounded sterile drugs purchased from outsourcing facilities.
The Pennsylvania Attorney General filed charges against a pharmacy owner and the Ohio Board of Pharmacy revoked licenses to protect patients.
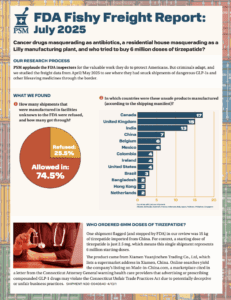
The newest Fishy Freight report continued to find suspect API shipped into the U.S. while a report from the United Nations’ Office of Drug Crime highlighted the dangers of contaminated and counterfeit medicines made with diethylene glycol and ethylene glycol.
Our podcast covers the latest in pharma crime and medicine safety.
Like your information on video? Subscribe to our YouTube playlist!

What did Ohio authorities find at this med spa?

Over 120 kgs of tirzepatide from unregistered overseas labs, and more.

A court case may have put the nails on the coffin for alternative funding programs.
Click the images below to see more recent videos.