News Coverage
The Partnership for Safe Medicines has been publishing information about the counterfeit drug problem around the world for more than a decade. With experts leading the organization and a committed and passionate set of writers and editors, our content is more in-depth than many other sources, which simply copy links to the news from other websites.
The former owner of a California hospital has pleaded guilty to participating in an illegal medical kickbacks conspiracy, and a medical equipment manufacturer has recalled of all of its spinal fusion hardware due to “performance failures that could cause patient harm.” The case is linked to more than two dozen lawsuits filed alleging that counterfeit…
Special Agent Burke shared cases such as grandmother Betty Hunter’s run-in with counterfeit cancer medication.
NAPB finds only 4% of online drug sellers meet United States safety standards: uncontrolled substances still readily available without a prescription online. For 6 years the National Association of Boards of Pharmacy (NABP) has surveyed online drug sellers to investigate industry trends and has published their findings in their Internet Drug Outlet Identification Program Progress…
New Scientist’s Curtis Abraham Extols Public Health Experts to Try Harder to Beat Fake Antimalarials
Author Curtis Abraham cites the latest results from the WorldWide Antimalarial Resist Network as motivation to improve malaria drug quality. Their most recent survey found that 30% of malaria drugs tested globally failed quality assurance tests. In an opinion piece for New Scientist, Curtis Abraham asks medical and disease professionals to work harder to stem…
FDA warns Natural Solutions Foundation that the products they offer for sale are not Ebola cures and are therefore “misbranded.” At present there is no practical cure for Ebola, though several different vaccines are in development. A company in New Jersey using Youtube, Facebook and other online means to advertise their offerings has been sent…
Download BlackMarketCancer_singlepage.pdf (881.4K) Ozkan Semizoglu earlier had pleaded guilty to charges that he and his company, Ozay Pharmaceuticals, shipped counterfeit versions of life-saving cancer medications into the United States. Ozay Pharmaceuticals was one of several sources for the fake cancer drugs that have plagued U.S. oncology clinics since 2012. The Department of Justice (DOJ) has…
Following earlier successful raids by the Ventura County Task Force, new investigations in Los Angeles County have turned up counterfeit and misbranded medications for sale at small stores and swap meets all over the LA. Basin. The Los Angeles Daily News reports that fake medication sellers working at stores and swap meets in Los Angeles…
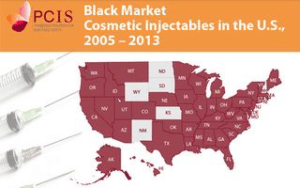
Between 2005 and 2013, there was one death and 11 other patients who required medical attention for disfigurement or serious illness as the result of receiving fake or misbranded cosmetic injectables while at a doctor’s office, clinic, or salon. Black Market Cosmetic Injectables in the U.S. 2005-2013 attempts to describe the scope of the problem.
UC San Diego research team develops a system by which automated web crawling programs can identify spam-based sales websites and connect them to large affiliate networks. A 2014 paper by UC San Diego researchers Matthew Der, Lawrence K. Saul, Stefan Savage, and Geoffrey M. Voelker titled “Knock It Off: Profiling the Online Storefronts of Counterfeit…
Wisconsin pharmacist and former Wisconsin State Pharmacy Board member, Marla Ahlgrimm, and her alleged co-conspirator Balbir Bhogal are facing a renewed indictment from their 2010 counterfeit medication importation case. The pair are alleged to have imported more than 4 million fake pills into the United States to fill the orders for a Costa Rican online…
3 Texas residents are alleged to have received more than 30 shipments of fake medications and acted as drop-shippers for a drug counterfeiting operation run from China. Counterfeit versions of painkillers and lifestyle drugs were among the seized fakes. The Department of Justice (DOJ) has announced the indictment of three Texans on counterfeit drug and…
Links between counterfeit medication production and organized crime illuminated as European authorities discover counterfeit medication producing equipment in a Europe-wide breakup of a child-trafficking ring. Over 1,000 arrested and 30 trafficked children freed as part of Operation Archimedes. Europol has just announced the largest ever cooperated international law enforcement operation that targeted organized crime specializing…
In an October 6th op-ed piece for The Hill, Partnership for Safe Medicines board member Jim Dahl described the growing threat that counterfeit drugs pose to U.S. patients, and asks Congress and the Federal Government to step up efforts to stop the spread of counterfeit drugs and fake online pharmacies. Jim Dahl, a retired Assistant…
Three Athens, Texas residents, Wanda Hollis, 63, Tom Giddens, 57, and Catherine Nix, 41 have been charged with one felony count of conspiracy to smuggle merchandise into the United States, seven counts of causing the introduction of misbranded drugs into interstate commerce with the intent to defraud or mislead, seven counts of smuggling and one…
The World Customs Organization and the International Institute of Research Against Counterfeit Medicines joined forces in Africa to try and stem the tide of counterfeit drugs sweeping though African nations. With Operation Biyela 2 customs agents in 14 African countries have conducted seizures that paint a clearer picture of how counterfeit medication makes its way…
A new report in the Journal of the American Medical Association examines how suspicious prescriptions for HIV medications indicate that HIV drugs are being diverted for resale on the black market. The September 17th 2014 issue of the Journal of the American Medical Association (JAMA) reports that HIV medication is being purchased by devious means…
The FDA has a new flyer campaign that asks doctors, medical office managers, and others responsible for medication purchasing to ensure that they only buy FDA-approved medication. Only state-licensed wholesale distributors of medication can guarantee the safety of medication prescribed to patients. The Food and Drug Administration (FDA) is asking all medical professionals to verify…
Tennessee-based IT manager Nancy White began digging deeper into EBay medication sales practices after receiving a dangerous pharmaceutical product in lieu of the skin cream she had ordered. According to Mississippi Attorney General Jim Hood, who spoke at the Partnership for Safe Medicine’s annual conference last week, there is a broad spectrum of illicit or…
Four members of the same Los Angeles area family have been indicted on counterfeit goods trafficking and mail fraud conspiracy charges for their sale of unapproved ED medications. Learn how drug counterfeiters are targeting Americans at Interchange 2014, September 18th in Washington D.C. Four members of the Gitmed family, John Gitmed, his ex-wife Holly, their…
Daniel Burke Special Agent at FDA-OCI tells Fierce Pharma about the FDA’s “Most Wanted” campaign. Effort highlights fugitives from justice for crimes ranging from posing as doctors to importing and distributing counterfeit medications. Come here Special Agent Daniel Burke speak September 18 in Washington D. C. at Interchange 2014. In a recent interview with Fierce…
Libby Baney of the Alliance for Safe Online Pharmacies and Gaurvika Nayyar of the U.S. Pharmacopeial Convention will speak September 18 at Interchange 2014 in Washington D.C. The Alliance for Safe Online Pharmacies (ASOP) announced on August 21st that the U.S. Pharmacopeial Convention (USP) is joining forces with them to stem the tide of counterfeit…
Two brothers, their mother and a cousin are accused of operating a large-scale fake online pharmacy scam that shipped to consumers all over the globe. The danger that fake online pharmacies pose to U.S. consumers is one of the primary topics we will be discussing September 18 at Interchange 2014 in Washington D.C. Trib Live…
Sample of drugs confiscated in EU raid. Photo Credit: Europol. Law enforcement across Europe dismantled a vast counterfeit medication ring. Two men in United Kingdom among several arrested for selling counterfeit medication online. Europol has posted a press release concerning a counterfeit drug investigation conducted in cooperation with investigators in Austria, Belgium, Cyprus, France, Hungary,…
In addition to charges filed last month alleging FedEx assisted fake online pharmacy businesses, new allegations charge FedEx acted to launder funds for known fake online pharmacies. The FedEx Corporation was indicted August 15 on additional charges that they participated in a criminal conspiracy to launder money for illegally operating fake online pharmacies, according to…
The move is an effort to bring to an end shareholder litigation concerning Google permitting advertising from illegal online pharmacies. Litigation was started in 2011 after Google settled with the U.S. government concerning use of Google Ad Words by fake online pharmacies. Reuters is reporting that Google has made a settlement with its shareholders to…
Six people have been indicted and Joaquin Grocery in the Bronx is shuttered after investigation alleges store was purchasing various prescription drugs from impoverished locals for resale to street users and local pharmacies. A Bronx New York grocery has been closed and several people involved in its operation indicted on charges the grocery store operated…
Sabahaddin Akman, owner and manager of Turkish drug wholesaler Ozay Pharmaceuticals pleaded guilty to counterfeit drug charges in Federal Court. Ozay Pharmaceuticals was one of the foreign-based drug exporters responsible introducing counterfeit cancer medications into the secure U. S. drug supply. Turkish citizen Sabahaddin Akman pleaded guilty to charges of smuggling misbranded and adulterated cancer…
This year, online pharmacies operating within the European Union will be able to demonstrate their compliance with local laws and regulations by displaying a new logo created to identify safe online pharmacies. Regulatory agencies within each country will be responsible for authorizing valid online pharmacies that operate within their borders. A new logo has been…
CDC researchers describe the growing problem of in-hospital infections in the United States being caused by drug thefts and contaminated syringe re-use by hospital employees. In the last 10 years over 30,000 patients have been exposed to pathogens via drugs diverted by infected medical professionals. A new report by CDC researchers Melissa K. Schaefer, MD,…
FDA Deputy Commissioner Howard Sklamberg, keynote speaker at Interchange 2014, has offered a clear explanation of FDA’s implementation of the Food and Drug Administration Safety and Innovation Act Title VII drug supply chain provisions. On July 9, 2012, President Barack Obama signed the Food and Drug Administration Safety and Innovation Act (FDASIA) in to law.…
Andrew Strempler, third from left, holds pharmacy orders. Jan 3, 2002 photo used with permission from the Canadian Press. The pharmacist in the Bahamas who provided order fulfillment for fake online pharmacy pioneer Andrew Strempler and his RXNorth/Mediplan online pharmacies has been ordered by an appeals court in the Bahamas to return to court. Missouri…
Jim Hood, Attorney General for the state of Mississippi and head of the National Association of Attorneys General tells Bloomberg that he is stepping up demands from Google that they demonstrate their commitment to block fake online pharmacies. Bloomberg reports that Attorney General Jim Hood has demanded information from Google to determine if the company…
A director of Ozay Pharmaceuticals, the Turkish drug distributor responsible for one of the infiltrations of counterfeit cancer medication into the secure U.S. drug supply chain, has pleaded guilty to charges related to the importation of counterfeit Avastin and other counterfeit cancer medications into the United States. Turkish national Ozkan Semizoglu pleaded guilty to charges…
Fayez Al-Jabri of Chicago acted as a black market wholesaler, importing thousands of doses of fake pills from China for further distribution. A Chicago resident has been sentenced to 3.5 years in prison for his role in importing for sale over 26,000 doses of counterfeit ED medication into the United States, reports the Department of…
FedEx N101FEsby Drewski2112 via Flickr. A nine-year investigation has culminated in indictments of FedEx companies for conspiracies to traffic in controlled substances and misbranded prescription drugs associated with illegal Internet pharmacies. Although warned by Congress as early as 2004, the FEDEX Corporation allegedly continued to act as a delivery service for fake online pharmacies, and…
Lawrence Stowe and his co-conspirator Francisco Morales lured desperate patients with ALS and other neurological ailments to their clinic with promises of miracle cures. Instead unsuspecting patients were subjected to untested and bogus treatments that cost as much as $100,000 per patient. Lawrence Stowe, the so-called doctor referred to as a “21st Century snake oil…
Confiscated fake drugs. Image courtesy of Ventura County Sheriff's Department. A tip called in to the local “Crime Stoppers” line informed the Ventura County Interagency Pharmaceutical Crimes Unit (VCIPCU) that fake drugs imported from Mexico were being sold in a local store in Santa Paula. Subsequent investigations led the VCIPCU to make arrests of 20…
Professor Dora Akunyili. Image courtesy of doraakunyilionline.org. Dr. Akunyili spearheaded Nigeria’s National Agency for Food and Drug Administration and Control (NAFDAC) transformation into a modern food and drug enforcement agency, and was tireless in her fight against counterfeit medication in Nigeria. After a two-year battle with cancer, Nigeria’s Professor Dora Akunyili passed away on June…
A doctor in Texas sentenced to probation, and an oncology practice office manager in Kentucky has pleaded guilty to charges that they imported non-FDA approved cancer medication. Quality Specialty Products, the Canada Drugs-owned pharmaceutical wholesaler responsible for the importation of counterfeit Avastin, was implicated in both cases. In Laredo, Texas Dr. Eduardo Miranda was sentenced…
Full infographic available from the World Health Organization. The explosion of the global counterfeit drug trade has magnified antimicrobial resistance for ailments such as malaria, tuberculosis, HIV, diarrhea, and pneumonia. Now public health experts in the United Kingdom are calling for an organization bring together global expertise to fight the threat of drug resistance. The…
The National Crime Prevention Council has 7 tips for buying prescription drugs online. 1. Never purchase prescription drugs without your doctor's prescription. 2. Be familiar with the mediations you take, including the color, size, shape, taste and side effects. 3. Make sure the package or container the medication comes in has not been altered or…
The European Medicines Agency (EMA) has reported two additional cancer treatments that have been illegally distributed, after their theft in Italy in April, 2014. Lots of two additional cancer medications, Avastin (bevacizumab), an injectable cancer treatment used for colorectal cancer, lung cancer, and kidney cancer and MabThera (rituximab), a treatment of cancers of the lymphatic…
Dr. Yashica Robinson-White, 38 of Opelika, Alabama is being prosecuted based on her business dealings with a black market wholesaler running out of Great Neck, New York. Dr. Robinson-White is accused of committing health care fraud for purchasing non-FDA approved IUDs from Medical Device King and implanting them in patients as if they were FDA-approved…
via Flickr. Canadian online pharmacy CanadaDrugs.com suspended from supplying drug wholesalers and pharmacies after Health Canada investigations produced “significant concerns” with their shipping and storage practices. Canada Drugs has previously been identified as the owner of several subsidiary companies in the United States and United Kingdom that have been implicated in the importation of counterfeit…
At the end of June in 2013, the FDA sent warning letters to close to 800 medical practices throughout the United States concerning their business exchanges with a black market medication wholesaler called Medical Device King/Pharmalogical. The subsequent investigation of Medical Device King has resulted in counterfeit drug and fraud charges being leveled against the…
At Interchange 2013, we heard from Christopher Weaver of the Wall Street Journal about the role black market drug wholesalers played in the distribution of counterfeit Avastin. Dr. Marv Shepherd also described the tactics shady drug wholesalers use to sell their wares to hospitals. Since then there have been several prosecutions against black market drug…
Washington, D.C. (June 5, 2014) – Marv Shepherd, President of the Partnership for Safe Medicines (PSM), the leading advocate in the fight against counterfeit prescription drugs, today issued the following statement on the 16th Global Anti-Counterfeiting Day: “On World Anti-Counterfeiting Day, PSM stands united with our global partners in the war against fake medicines. Although…
In another case of a medical practitioner stepping outside the secure drug supply chain, a Florida doctor stands accused of purchasing medication on the black market via Internet sites purporting to be in Canada and elsewhere. Howard Skalmberg, Deputy Commissioner for the FDA’s Global Regulatory Operations and Policy, will be the keynote speaker at Interchange…
Photo Credit: Interpol Operation Pangea, Interpol’s annual cooperative effort with law enforcement and customs agents from throughout the globe, targets fake online pharmacies and the drug-counterfeiting criminals that run them. In its seventh year, Operation Pangea VII shut down over 10,000 fake online pharmacies, and seized 9.4 million doses of fake drugs. On May 22,…
In May 2014, the Community Access National Network (CANN) and the Partnership for Safe Medicines (PSM), released a new resource for the HIV/AIDS community and their doctors. The flyers, available online and in print form, highlighted the incidence of black market HIV/AIDS medication since 2006 and offered tips for patients and physicians to ensure they are purchasing legitimate drugs from reputable sources.
Internet purchases of prescription drugs proliferate in Canada, exposing unsuspecting Canadians to black market and counterfeit medication. At Interchange 2014, we’ll tackle the current state of fake online pharmacies and help you decode fake pharmacy websites. In part one of a two-part series on Canadian fake online pharmacies, iPolitics describes the trend among Canadian consumers…
Syed “Farhan” Huda, co-owner of Gallant Pharma International, is one of 12 people convicted for their roles in operating a vast unlicensed pharmaceutical wholesale operation from suburban Virginia. Last year, Huda pleaded guilty to illegal importation, introducing misbranded drugs, unlicensed medical wholesaling and wire fraud. Five months after pleading guilty to charges that he was…
Italian Medicines Agency representative identifies the powerful Camorra organized crime family as the alleged ringleaders of medicine thefts, with five truckloads of drugs going missing a month. European authorities recovered lots of the stolen cancer injectable drugs where the genuine medication had been replaced with low-cost antibiotics. The Wall Street Journal reports that the recent…
Board votes unanimously to ask Maine Attorney General to send cease-and-desist letter to a Canadian online pharmacy, and to investigate the charge that they are violating Maine law. CanadaDrugCenter’s activities first came to light when Maine Pharmacy Association President Kenneth McCall filed a complaint against them for selling medication that did not originate in approved…
New infographic focuses on dangers to women’s health from counterfeit and black market medicine Washington, D.C. (May 14, 2014) – This week is women’s health week, and nationally-known patient advocates Men’s Health Network and public health advocates the Partnership for Safe Medicines (PSM) are sounding the alarm about the growing danger of counterfeit and black…
Fight The Fakes Infographic for Europe
In honor of Women’s Health Week, we have partnered with Mens Health Network to share with you the “9 Risks to Women’s Health From Counterfeit Drugs” By S. Imber
At Interchange 2013 we were fortunate enough to have Andy Shuttleworth of the National Intellectual Property Rights Coordination (IPR) Center speak about their work fighting the dangers of fake online pharmacies. At Interchange 2014 on September 18th, we will present a panel of experts in the field who will discuss the state of the fake…
From chainsaws that come apart while in use to medications that don’t work or make people sick, products for sale online are increasingly being found to be counterfeited. An April 30th report in USA Today points out that dangerous fake goods are easily found for sale on the Internet, and counterfeit medication numbers among the…
FDA Deputy Director Bernstein Shares What FDA is Doing To Enforce the Drug Supply Chain Security Act
Deputy Director of the Office of Compliance in FDA’s Center for Drug Evaluation and Research Ilisa Bernstein, describes what efforts the FDA is making to enact the drug safety requirements of the Drug Supply Chain Security Act (DSCSA), and invites stakeholders in the fight against counterfeit drugs to answer questions regarding the security and safety…
Have you registered for our annual conference on September 18th? Don’t miss the 2014 Interchange with keynote speaker Howard Sklamberg, FDA’s Deputy Director. Early bird discounted registration is still available! Click here to register today. Attorneys general from several states want to make it more difficult for search engine users to find fake online pharmacies using…
2.4 million counterfeit pills were seized by French customs during routine search of a shipment from China. Fake versions of aspirin, diarrhea treatment & ED medications were being shipped disguised as packages of Chinese tea. French customs has notified the media that a huge haul of fake drugs worth about $1.38 million was seized by…
European Medicines Agency released warning that previously stolen vials of Herceptin have been repurposed as counterfeit versions of the drug. Lots of the adulterated cancer drug have been found in the United Kingdom, Germany and Finland. Swiss pharmaceutical manufacturer Roche has recalled several lots of its breast cancer treatment, Herceptin, reported Bloomberg News on April…
This is a reprint of an FDA Alert. Recall — Firm Press Release FDA posts press releases and other notices of recalls and market withdrawals from the firms involved as a service to consumers, the media, and other interested parties. FDA does not endorse either the product or the company. Contact:Consumer:1-714-515-4600nanowellbeingh@gmail.com FOR IMMEDIATE RELEASE –…
Kenneth McCall of the Maine Pharmacy Association wanted to find out what he would receive when ordering medication from Canada Drug Center, a company who began advertising in Maine as soon as the new drug importation law was passed. When he saw the drugs were sourced in places not permitted by the new law, he…
EDQM collects counterfeit drug cases that have impacted Europe into database termed KNOW-X, in an effort to assist investigators seeking to stem the tide of counterfeit medicines pouring into the European Union. The European Directorate for the Quality of Medicines and Healthcare (EDQM) has just announced their latest effort to combat counterfeit drugs entering the…
Royal Pharmaceutical Society and UK morning show Daybreak surveyed 2,500 UK pharmacists on fake online pharmacies. Results show counterfeit drugs and fake online pharmacies are a growing threat to patients in the United Kingdom as well as the United States. A 2013 survey of pharmacists in the United Kingdom found that UK patients are exposing…
The Wall Street Journal reports Ozay Pharmaceutical Co. in Istanbul as a source of counterfeit cancer medication that ended up in U.S. doctors’ offices. Two men who worked there, Ozkan Semizoglu and Sabahaddin Akman are currently charged with smuggling illicit cancer drugs into the United States. A Turkish drug exporter is at the center of…
iPolitics Investigates How Drug Counterfeiters Are Using Craigslist to Hawk Their Wares to Canadians
Annie Marie Oliver describes how the growing problem of counterfeit drugs being offered for sale on Craigslist is presenting serious challenges to Canadian law enforcement. A story in iPolitics is reporting that counterfeit drugs are easily available to Canadians via sellers on Craigslist. Titled “Craigslist: Increasingly, a marketplace for dodgy prescription drugs”, author Annie Marie…
The Partnership for Safe Medicines teamed up with The Doctors Company, the country's largest malpractice group, to educate doctors on this key issue at a time when prosecutions of physicians are increasing. Click here to read. If you can help us reach physicians, we would love to spread the word on this important issue to…
In Hidalgo, Texas, three different beauticians are accused of injecting patients with toxic, non-medical silicone or other liquid plastics instead of an FDA-approved dermal filler. In all, 30 patients report health issues, and at least one has died. On the 19th of the month, Elva Navarro, owner of Bella Face and Body Spa in McAllen,…
Download BlackMarketCancer_Long.pdf (881.4K) William Burdine, owner of Alvarado Medical Plaza Pharmacy in San Diego, had earlier pleaded guilty to charges he purchased injectable cancer medication from Quality Specialty Products (QSP) of Canada. QSP first came to the attention of Federal authorities when they were identified as a source of counterfeit Avastin. Alvarado Medical Plaza Pharmacy…
Investigators turn up over $2 million worth of counterfeit health and beauty products, including counterfeited cold medicines and painkillers. Nassau County prosecutors allege that two brothers, Pardeep Malik and Hamant Mullick ran a multi-state health and beauty product counterfeiting business, CNN reports. According to CNN, “Law enforcement authorities seized four tractor-trailers filled with knockoff health…
Photo Credit: CDC Fluview This year’s influenza epidemic has affected thousands. Over 2,700 influenza positive tests have been reported as of March 7th for the 2013-2014 season reports the CDC. As flu season slowly comes to a close, drug counterfeiters are still racing to market their fake flu cures, which will not cure influenza. Don’t…
Kamran Rezapour of Creston, North Carolina admitted that he knew the herbal supplements he sold contained pharmaceutical compounds not described in the ingredients. However he described them as “100% all natural” in his marketing materials. A Creston, North Carolina man has pleaded guilty to knowingly selling tainted natural supplements smuggled into the United States from…
A doctor, a woman who posed as a doctor, and the manager of operations at Manor Medical Imaging were found guilty of federal fraud charges as part of an organized scheme to repeatedly bill Medicare and Medi-Cal for thousands of prescriptions, including expensive anti-psychotic treatments. One of the largest Medicare fraud cases to be heard,…
The Partnership for Safe Medicines is pleased to announce the keynote speaker for Interchange 2014 on September 18, in Washington DC: Howard Sklamberg, Deputy Commissioner for the FDA’s Global Regulatory Operations and Policy. Deputy Commissioner Sklamberg directs the Office of Regulatory Affairs and the Office of International Programs. He oversees the FDA’s efforts to supervise…
In his op-ed featured in The Hill on March 1, 2014, LaGanga anticipated that the recent hearing Counterfeit Drugs, Fighting Illegal Supply Chains will spark new efforts by the government to educate patients and take stronger action to stop this growing health threat. He encouraged policymakers to better understand the real nature of the threats posed…
Photo Credit: Pfizer Corporation. Decision by European Parliament gives front-line customs agents the power to seize suspected counterfeit medications that cross their border even if they are in transit to a different country. The European Parliament passed a draft law making it easier for customs authorities to intercept counterfeit medications that pass in transit between…
Washington, D.C. (February 27, 2014) – Marv Shepherd, President of the Partnership for Safe Medicines (PSM), released the following statement on today’s hearing on counterfeit medicines held by the House Energy and Commerce Committee’s Subcommittee on Oversight and Investigations. The hearing, titled “Counterfeit Drugs: Fighting Illegal Supply Chains” brought to light some disturbing trends that must…
In Online Availability And Safety Of Drugs In Shortage: A Descriptive Study Of Internet Vendor Characteristics, Dr. Tim Mackey and Dr. Bryan Liang examined how Internet pharmaceutical vendors take advantage of known drug shortages to market unapproved and unsafe versions of these medications directly to consumers. Find out why drug shortages play into the hands…
Black market medicine distributors have impacted patient health in many areas. We examined two different therapeutic areas of non-FDA approved medication infiltrating the secure U.S. drug supply chain. Learn more about the prescription drug black market and what policy makers, medical professionals, patients and consumers can do to protect the secure U.S. drug supply chain.…
Take a moment to peruse “Are Tier One Countries Safe to Import Medicine From?” and teach yourself about the true dangers inherent in medicine importation. The Partnership for Safe Medicines answers the question of drug importation safety with a resource designed to make clear the real costs you incur when you purchase non-FDA approved medications from…
The Partnership has released a new version of this publication. Please consult the 2017 edition here. Download and read Counterfeit Drugs: A dangerous threat to American patients. This resource developed by The Partnership for Safe Medicines explains the counterfeit medicines issues as they impact the U.S. Explaining the breadth of the problem, it then details the…
An illicit drug lab operating in Longueuil, Canada was outfitted to produce thousands of pills. Alleged lab owner, Alexandre Beaudry is scheduled to appear in court on charges relating to these operations on February 20, 2014. The Royal Canadian Mounted Police (RCMP) reported that their Clandestine Laboratory Enforcement Team (CLET) have dismantled a counterfeit drug…
When CBS Dallas reported on prescription fulfillment errors in Texas pharmacies, they reached out to The Partnership for Safe Medicine’s Board President, Dr. Marvin D. Shepherd to discuss the prevention of dispensing errors. Dr. Shepherd is Director of the Center for Pharmacoeconomic Studies and Chairman of the Pharmacy Administration Division at the University of Texas…
Download the 3 Steps to Safety here. On January 29, 2014, the U.S. Department of Justice (DOJ) announced that seven Ohio oncologists had been sentenced to probation for purchasing cancer medications for patients from a foreign supplier. Each doctor pleaded guilty to causing the shipment of misbranded drugs, a misdemeanor, reports the DOJ. In each…
Download HeartSmart5.pdf (1142.8K) Download and share “Be Heart Smart About Natural Supplements” which explains the dangers that can be posed to your heart by seemingly innocuous “natural” dietary supplements. A quick visit to the FDA Consumer page devoted to dietary supplements demonstrates only too clearly the dangers posed by these untested and unregulated “natural” treatments.…
Two Turkish nationals have been indicted in a counterfeit cancer drug smuggling ring that illegally imported fake cancer treatments from Turkey and other countries and sold them to doctors and clinics in the Saint Louis area. Ozkan Semizoglu and Sabahaddin Akman, both citizens of Turkey, are alleged to have imported “unapproved, misbranded, adulterated and counterfeit…
FDA Special Agent Dan Burke was gracious enough to participate in an Interchange 2013 panel concerning fake online pharmacies. Special Agent Burke knows more about fake online pharmacies than most. His job is to investigate the highly organized illegal operations behind a typical fake online pharmacy. He is the senior operations manager in FDA’s Cybercrimes…
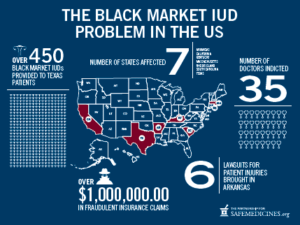
Between 2009 and 2014, 35 doctors in 7 states were prosecuted for exposing women to non-FDA approved IUDs. Doctors have been accused of purchasing IUDs from “Canadian” fake online pharmacies and importing untested IUDs from Mexico, then billing Medicare for the cost of genuine, FDA-approved IUDs. Over the same time period, authorities estimated that over 450 women were implanted with misbranded, black-market IUDs.
Learn more about black market cancer drug cases by downloading this resource. While many pharmacists act as custodians for their patients’ safety, three different pharmacists in California, New York, and Pennsylvania have either been indicted or pleaded guilty to charges ranging from prescription drug smuggling and money laundering, to heath care fraud and illegal diversion…
Graphic from PwC’s report, “Younger consumers are more concerned about the safety and quality of their medications” A recent survey by Pricewaterhouse Coopers found that people 18-24 had the greatest concerns when it comes to medicine safety. Greater numbers of young consumers express concern about the quality and safety of their medications than those 65…
A news channel conducted their own investigation into purchasing prescription drugs online. Testing of the drugs they received proved they were counterfeit, and in some cases contained dangerous banned compounds. A Colorado news station, 9 News Colorado, shared with their viewers the disturbing experience of their sister news station, WKYC in Cleveland. WKYC’s investigation in…
Download What Kind of Businesses Are Fake Online Pharmacies New campaign, “Counterfeit: Don’t Buy Into Organized Crime” addresses the dangers posed to consumers who purchase medicine that turns out to be counterfeit. “Counterfeit: Don’t Buy Into Organized Crime,” the new consumer awareness campaign launched today by the United Nations Office on Drugs and Crime…
Download BlackMarketCancer_Long.pdf (881.4K) Dr. Eduardo Miranda, an oncologist practicing in Laredo, Texas, was accused of illegally importing non-FDA approved cancer medications from a known source of counterfeit Avastin. In December 2013, the U.S. Department of Justice (DOJ) reported that Dr. Eduardo Miranda had pleaded guilty to charges that he imported non-FDA approved cancer medication into…
Ms. Arnold spoke at Interchange 2013 about the events in her state that resulted in the passage of a controversial law that allows non-FDA approved medication to be imported from unregulated sources into the state of Maine. At the heart of this recent law is a Canadian business called CanaRX that Ms. Arnold stated “came into…
In August 2013, the Department of Justice announced indictments of eleven people involved in running an unlicensed pharmaceutical wholesale business out of suburban Virginia. To date, seven employees of Gallant Pharma International, along with one of its co-owners have pleaded guilty to charges of importing and distributing non-FDA approved injectable medications via their unlicensed wholesale…
Download FSA.pdf (493.3K) As we approach the end of the year, people seeking to empty their flexible spending accounts or avoid new prescription deductibles in the new year will order prescriptions and other medical supplies online.But the convenience of purchasing online shouldn’t prevent you from ensuring that your…
The Federal Bureau of Investigation announced on December 11, 2013 the indictment of Salim Yusufov, owner and operator of Healthway Pharmacy in Pikesville, MD. Yusufov is charged with illegally providing unapproved prescription drugs for customers from Germany and Eastern Europe. The medication, Corvol, contains large amounts of phenobarbital, and is used to treat elevated blood…